
Facility type and cancer outcomes in the United States
Introduction
Cancer has emerged as a significant public health problem worldwide, and trends suggest that low- or middle-income countries (LMICs) will experience a substantial increase in cancer burden by the year 2040 (1). In the United States (U.S.), over 1.8 million people are diagnosed annually with cancer, and nearly 610,000 people will die from their disease in 2023 alone (2). Despite recent advances in cancer diagnosis and therapy, the prognosis for many malignancies remains poor, and this is particularly true for certain racial and ethnic groups, who often see increased cancer incidence, prevalence, mortality, and disease burden (3-5).
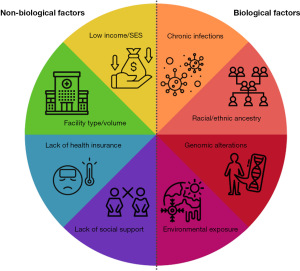
Genetic and biological risk factors contribute to many different cancer health disparities. Biological factors include racial or ethnic ancestry, genomic or cytogenetic alterations, chronic infections, and environmental exposures involving birthplace. However, there are also non-biological factors that play a role in cancer risk and outcomes. Non-biological factors such as socioeconomic status (SES), lower education or income levels, lack of health insurance, and geographic location can restrict health care access (5). Thus, improved access to care for underserved populations could dramatically affect cancer incidence rates and outcomes.
Importantly, sociodemographic factors are linked to both health care quality and delivery, and can influence where a patient receives cancer diagnosis and treatment. In this editorial, we reflect on the status of cancer health disparities in the U.S., and how they are impacted by facility type and volume in underserved minority populations.
Factors influencing cancer health disparities in the U.S.
Health disparities are defined as variations in the incidence, prevalence, mortality, and burden of diseases among specific population groups. Cancer health disparities describe the differences in cancer outcomes among those various groups. These are measured in terms of cancer incidence, prevalence, stage at diagnosis, time to treatment, morbidity and mortality, screening rates, survivorship, and quality of life. The populations most often affected by health disparities are minority groups, defined as a subgroup with unique social, religious, ethnic, racial, or other characteristics that differ from the majority. This includes groups subjected to oppression or discrimination by those in more powerful positions, regardless of whether the group is a numerical minority.
Cancer health disparities affecting diverse U.S. populations result from an interplay between numerous different biological and non-biological factors. Health disparities research has shifted from single-dimension models to more complex frameworks that incorporate a number of factors, including biological, behavioral, physical environmental, sociocultural environment, and health care systems. Here, we briefly outline some known biological and non-biological factors contributing to cancer health disparities in U.S. minority populations.
Biological factors
Substantial evidence suggests that the unequal cancer burden among minority populations can be partially explained by genetic ancestry (6). Cancer is a genetic disease, and genome-wide association studies (GWAS) have discovered loci associated with increased risk. However, while genomic changes could explain the etiology behind any given cancer, it fails to paint the entire picture regarding cancer health disparities. The slow rate of progress in cancer health disparity research is partly due to the lack of data, biospecimens, and funding available to study diverse populations.
In addition to genomic alterations, some cancer health disparities are attributed to differential incidences of certain diseases by race or ethnicity. In utero cytomegalovirus (CMV) infection leads to the development of childhood acute lymphoblastic leukemia (ALL). Hispanic children are disproportionately affected, with nearly six times the risk for blast cell conversion to ALL compared with non-Hispanic White children (7). Another example is hepatocellular carcinoma (HCC), with hepatitis C as one of the primary drivers of tumor formation. Black and Hispanic patients are less likely to have opportunities to undergo curative antiviral therapy (8).
Studies also suggest that cancer incidence and mortality in diverse populations can be further stratified by place of birth. For instance, in breast cancer and prostate cancer, incidence and survival differ among Latin American countries (9). Differences have also been documented for populations of African Americans/Blacks, including U.S.-Blacks, Jamaicans, and Haitians (10). These data suggest that behavioral practices (e.g., reproductive behavior including mode of delivery and breastfeeding, changes in diet, smoking) or environmental exposures that are more common in the U.S. could lead to biological differences that affect cancer outcomes.
Non-biological factors
Low SES patients tend to present later in their disease progression, likely due to barriers for health care utilization, including delays in screening, diagnosis, and appropriate treatment (11). Insurance status is also strongly correlated with health outcomes, and patients without health insurance or on Medicaid have higher mortality rates compared to those with private insurance (12). This survival disparity is also present between married and unmarried populations, with married patients having better overall survival than unmarried (including single, divorced, and widowed) patients (13). Patients living in rural areas, along the U.S.-Mexico border, and long distances from a comprehensive cancer center likewise tend to have worse prognoses (4,5,14). In all of these groups (low income, low education, uninsured, unmarried, and rural or border population), patients are more likely to present later in their disease progression. They are less likely to receive treatment for their conditions compared with high-income, educated, privately insured, or married patients (15). Approaches to combat these observed cancer health disparities should focus on the complex interaction between both biological and non-biological factors (summarized in Figure 1).
Facility type and cancer outcomes in the U.S.
In addition to the biological and non-biological factors listed above, characteristics of the treatment facility also impact survival. Indeed, adult cancer patients who received initial therapy at a National Cancer Institute-designated Comprehensive Cancer Center (NCICCC) had superior survival compared with patients who received therapy at a non-NCICCC facility (16). Similarly, short- and long-term survival after complex cancer therapies were superior at top-ranked hospitals compared with affiliates of such institutions (17). Studies have shown a strong correlation between increasing patient volumes and better outcomes in many different diseases (18). For acute myeloid leukemia (AML) patients, mortality rates were significantly higher in low-volume compared with high-volume cancer centers (19). Another study showed that predictors of in-hospital death or discharge of AML patients to hospice included lower hospital volume combined with older age and geographic location (20). Additionally, AML patients treated in a community setting have worse outcomes than those treated at an academic center (21).
Hospital facilities have been shown by several groups to impact patient outcomes after cancer surgery (18). For cancers that can be treated with curative radiation therapy, treatment at a high-volume facility was associated with improved survival (22). It should be noted that cancers with the greatest benefit from high-volume facilities were those with the highest number of cancer-related deaths, including cancers of the pancreas, esophagus, and brain (23). Select studies have shown poorer survival at large institutions, possibly due to the complexity of patients referred to and treated at these centers (24).
A recent article published in Annals of Cancer Epidemiology outlined the impact of facility type and volume on outcomes for patients with AML (25). Their primary objective was to evaluate the impact of treatment facility type and volume on time to treatment. Their secondary objective assessed the impact of these same facility characteristics on overall survival, receipt of chemotherapy, allogeneic hematopoietic stem cell transplant, and/or receipt of palliative care. Altogether, they showed that low-volume facilities had a shorter time to treatment than high-volume facilities. However, the faster time to treatment observed at low-volume facilities did not lead to improved clinical outcomes. Overall survival was significantly higher at academic centers compared with all other facility types, and worse at low-volume compared with high-volume facilities. Patients at academic centers had a higher odds of treatment with chemotherapy and allogeneic stem cell transplantation, whereas low-volume facilities had a greater likelihood of referral to palliative care (25). Altogether, the authors demonstrated that high-volume facilities and academic centers had better overall survival, and a greater likelihood of treatment with chemotherapy and allogeneic hematopoietic stem cell transplantation. They further showed that Black race and Hispanic ethnicity correlated with differences in time to treatment, overall survival, and receipt of certain therapies, with largely worse outcomes (25). In general, they reasoned that resource-rich facilities have more experience with managing treatment-related complications, and have better access to novel treatment options in the form of clinical trials. These findings highlight the need to improve health care access at multiple levels, in order to reduce and eliminate cancer health disparities in U.S. minority populations. Further investigation is warranted to not only describe the cancer disparities based on treatment facilities, but also identify ways to address them. Financial barriers, health care economics, the inability to recruit qualified personnel, resistance to change or skepticism of novel treatments, and differences in the patient population could all play a role.
Concluding remarks
A combination of biological and non-biological factors contributes to cancer health disparities in U.S. minority populations, and a comprehensive approach to treatment and prevention is necessary. Biological factors include genetic ancestry, chronic infections, and environmental exposures involving birthplace. Non-biological factors such as education, income, insurance status, marital status, facility type, and volume are associated with differences in survival in many cancers, including blood cancers and solid tumors. While low levels of education, low income, being uninsured or on Medicaid, being unmarried, and treatment at low-volume facilities are each independently associated with worse outcomes, it seems that these factors compound on one another in a synergistic way. Additionally, these variables are more likely to occur together than they are apart. For example, married patients are more likely to have health insurance and have a higher household income. Less clear variables may also contribute to the differences in survival, especially for those associated with lower income. People with a lower income have different stressors, environments, and behaviors than those with a higher income.
Patients face numerous barriers when attempting to access health care, which is often magnified for low-income populations. The cost of health care is a barrier for many people, which is a limiting factor for accessing care. While lack of health insurance creates a large hurdle, some may experience hardship in paying for health care, even with insurance. Patients may delay care due to an inability to pay a copay or deductible. A lack of reliable transportation may also limit access to health care. Lower-income patients often rely on public transportation, carpooling, walking, or biking. Additionally, when receiving care at a hospital or clinic, patients must ask for time off from work and often need to find childcare during those hours. These factors may cause a delay in care, resulting in advanced disease at the time of diagnosis. We cannot forget the other variables that contribute to survival differences, including diet, exercise habits, stress levels, social relationships, language barriers, and feelings of control and autonomy. The end goal of achieving health care equity for all requires addressing these disparities with a consideration of both non-biological and biological contributors, from economic stability, education, health care access, and social support, to the environment, genomic alterations, ancestry lineage, infections, and facility type.
Acknowledgments
Funding: This work was funded in part by the American Cancer Society Research Scholar Grant (No. RSG-23-1025481-01-IBCD) (AM Eiring) and the Cancer Prevention and Research Institute of Texas, Texas Regional Excellence in Cancer Award (No. RP230420) (AM Eiring).
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Annals of Cancer Epidemiology. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ace.amegroups.com/article/view/10.21037/ace-23-4/coif). AME was funded by the American Cancer Society and the Cancer Prevention and Research Institute of Texas. Aside from grant funding, there is nothing to disclose. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA Cancer J Clin 2023;73:17-48. [Crossref] [PubMed]
- American Cancer Society. Cancer Facts & Figures for Hispanics/Latinos 2018-2020. Atlanta: American Cancer Society, 2018.
- Bencomo-Alvarez AE, Gonzalez MA, Rubio AJ, et al. Ethnic and border differences on blood cancer presentation and outcomes: A Texas population-based study. Cancer 2021;127:1068-79. [Crossref] [PubMed]
- Bencomo-Alvarez AE, Rubio AJ, Gonzalez MA, et al. Blood cancer health disparities in the United States Hispanic population. Cold Spring Harb Mol Case Stud 2021;7:a005967. [Crossref] [PubMed]
- Daly B, Olopade OI. A perfect storm: How tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and pro-posed interventions for change. CA Cancer J Clin 2015;65:221-38. [Crossref] [PubMed]
- Francis SS, Wallace AD, Wendt GA, et al. In utero cytomegalovirus infection and development of childhood acute lymphoblastic leukemia. Blood 2017;129:1680-4. [Crossref] [PubMed]
- Rich NE, Hester C, Odewole M, Murphy CC, Parikh ND, Marrero JA, et al. Ra-cial and Ethnic Differences in Presentation and Outcomes of Hepatocellular Carcinoma. Clin Gastroenterol Hepatol 2019;17:551-9.e1. [Crossref] [PubMed]
- Pinheiro PS, Callahan KE, Siegel RL, et al. Cancer Mortality in Hispanic Ethnic Groups. Cancer Epidemiol Biomarkers Prev 2017;26:376-82. [Crossref] [PubMed]
- Pinheiro PS, Callahan KE, Ragin C, et al. Black Heterogeneity in Cancer Mortality: US-Blacks, Haitians, and Jamaicans. Cancer Control 2016;23:347-58. [Crossref] [PubMed]
- Percy-Laurry A, Altekruse SF, Hossain MB, et al. Association Between Socioeconomic Status and Tumor Grade Among Black Men with Prostate Cancer. J Natl Med Assoc 2018;110:53-7. [Crossref] [PubMed]
- Ebner PJ, Ding L, Kim AW, et al. The Effect of Socioeconomic Status on Treatment and Mortality in Non-Small Cell Lung Cancer Patients. Ann Thorac Surg 2020;109:225-32. [Crossref] [PubMed]
- Eskander MF, Schapira EF, Bliss LA, et al. Keeping it in the family: the impact of marital status and next of kin on cancer treatment and survival. Am J Surg 2016;212:691-9. [Crossref] [PubMed]
- Dhakal P, Lyden E, Muir KE, et al. Effects of Distance From Academic Cancer Center on Overall Survival of Acute Myeloid Leukemia: Retrospective Analysis of Treated Patients. Clin Lymphoma Myeloma Leuk 2020;20:e685-90. [Crossref] [PubMed]
- Siegel RL, Wagle NS, Cercek A, et al. Colorectal cancer statistics, 2023. CA Cancer J Clin 2023;73:233-54. [Crossref] [PubMed]
- Wolfson JA, Sun CL, Wyatt LP, et al. Impact of care at comprehensive cancer centers on outcome: Results from a population-based study. Cancer 2015;121:3885-93. [Crossref] [PubMed]
- Boffa DJ, Mallin K, Herrin J, et al. Survival After Cancer Treatment at Top-Ranked US Cancer Hospitals vs Affiliates of Top-Ranked Cancer Hospitals. JAMA Netw Open 2020;3:e203942. [Crossref] [PubMed]
- Morche J, Mathes T, Pieper D. Relationship between surgeon volume and outcomes: a systematic review of systematic reviews. Syst Rev 2016;5:204. [Crossref] [PubMed]
- Giri S, Pathak R, Aryal MR, et al. Impact of hospital volume on outcomes of patients undergoing chemotherapy for acute myeloid leukemia: a matched cohort study. Blood 2015;125:3359-60. [Crossref] [PubMed]
- Zeidan AM, Podoltsev NA, Wang X, et al. Patterns of care and clinical outcomes with cytarabine-anthracycline induction chemotherapy for AML patients in the United States. Blood Adv 2020;4:1615-23. [Crossref] [PubMed]
- Levin A, Kleman A, Rein L, et al. Early mortality in patients with acute myelogenous leukemia treated in teaching versus non-teaching hospitals: A large database analysis. Am J Hematol 2017;92:E563-5. [Crossref] [PubMed]
- Tchelebi LT, Shen B, Wang M, et al. Impact of radiation therapy facility volume on survival in patients with cancer. Cancer 2021;127:4081-90. [Crossref] [PubMed]
- Stoltzfus KC, Shen B, Tchelebi L, et al. Impact of Facility Surgical Volume on Survival in Patients With Cancer. J Natl Compr Canc Netw 2021;19:495-503. [Crossref] [PubMed]
- Roman KM, Torabi SJ, Bitner BF, et al. The Impact of Facility Type and Volume on Outcomes in Head and Neck Mucosal Melanoma. Otolaryngol Head Neck Surg 2023;168:1079-88. [Crossref] [PubMed]
- Taylor AO, Doucette K, Ma X, et al. Facility type and volume impact outcomes in acute myeloid leukemia—a National Cancer Database Study. Ann Cancer Epidemiol 2023;7:2. [Crossref]
Cite this article as: Kilcoyne M, Nhim V, Gonzalez MA, Olivas IM, Eiring AM. Facility type and cancer outcomes in the United States. Ann Cancer Epidemiol 2023;7:4.


