Timely cancer diagnosis and treatment: towards a generalisable research framework studying timeliness to appropriate care
Importance of times to cancer diagnosis and treatment
Cancer is the second leading cause of death after cardiovascular diseases worldwide (1). To improve survival and quality of life from this life-threatening disease, early diagnosis and treatment are essential. Also, at the health system level, it is important to ensure sufficient capacity to deliver equitable and early accessible care through adequate workforce and facilities along the patient journey (2,3).
Internationally, many cancer control policies have included initiatives to improve the timeliness of diagnosis and treatment (2,4-6). In recent years, diagnostic and treatment delays have been further highlighted as a priority for government policies in light of the effects of the coronavirus disease 2019 (COVID-19) pandemic on cancer incidence and patient outcomes (3,7-11). Accordingly, measuring the time interval along diagnostic and care pathways has become key quality indicators (7,8). Risk factors associated with longer intervals are important to identify and interventions that aim to improve timely diagnosis and treatment are developed, contributing to an earlier stage at diagnosis and thus curable treatment options (e.g., surgery) (8). For example, countries offering cancer screening have found earlier stage at diagnosis and lower mortality among patients who participate (12). Yet, most patients, especially those with lung cancer (the leading cause of cancer death) (1), are not detected through cancer screening; instead, they are identified through self-referral, with self-appraisal and help-seeking, sometimes inevitably causing delays. For those with a later stage at diagnosis, the timing of using first-line and subsequent-line treatment modalities has become an important topic, due to the current availability of advanced treatment options including targeted therapies and immunotherapies (3). As reported, time intervals including the time to treatment initiation are various across settings, countries/regions and cancer types (7,8).
Current methodological frameworks to study times to cancer diagnosis and treatment
In recognition of the large number of cancer studies on diagnostic and treatment intervals as reporting the heterogeneity in methods used, there has been a call for standardised frameworks ensuring good quality of study design and reporting for robust and generalisable results. Specifically, these studies in this field are troubled by substantial variations in time intervals reported, as well as inconsistent or unclear definitions of these intervals (7,8,13,14). In one systematic review on times to diagnosis and treatment for lung cancer, a total of 96 unique time intervals were identified (14). Inconsistent or poor definitions were found for diagnostic and treatment intervals with different starting points or limited description of the starting point.
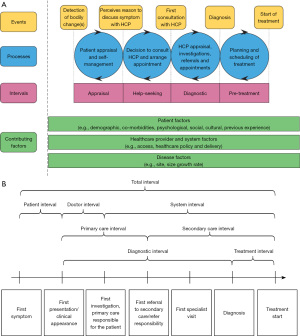
The Aarhus statement, published in 2012, made recommendations about defining key intervals along the pathway from the development of a cancer symptom to eventual diagnosis and treatment (15). The statement referred to two previously published papers that defined these intervals: “The Model of Pathways to Treatment” and a Danish model from Olesen et al. (Figure 1) (16,17). In both of these models, key milestones and the corresponding intervals along the patient journey have been carefully defined. Specifically, the patient interval (from first symptom to first presentation in clinical setting), diagnostic interval (from first presentation in clinical setting to diagnosis) and treatment interval (from diagnosis to treatment initiation).
There are some challenges though when to choose between either of these models. Firstly, both models were developed with an emphasis on countries where primary care has a key role in the initial assessment of symptoms and referral to hospital specialist care for diagnostic testing (e.g., Denmark, UK). This is especially true for the Olesen et al. model which explicitly defines the primary care interval. However, there are many countries with less developed primary care systems or where primary care does not act as a gatekeeper to hospital care (e.g., China, US). In these countries, patients can have direct access to cancer specialists. The Olesen et al. model is difficult to apply in such settings. The Model of Pathways to Treatment is potentially more flexible from this perspective as it includes a single diagnostic interval without trying to separate different health system components (primary vs. secondary care) where the diagnostic tests occur. This also allows greater flexibility when applying the framework for primary care systems where clinicians have greater access to diagnostic tests (8).
Another associated challenge in the implementation of the current frameworks relates to different routes to diagnosis and treatment. The emphasis of the frameworks is on symptomatic presentation, predominantly starting in primary care. However, the entry points in the pathways to diagnosis are complex and heterogeneous in the real world, including symptomatic presentation in emergency care, asymptomatic presentation via cancer screening, or incidental detection via routine care or investigation due to other disease (2,8,18). Specifically, cancer screening has become a key component within many patient journeys nowadays. Methodological frameworks need to be explicit on how to account for these other types of first presentation. Similarly, regarding the milestone of treatment commencement, cancer treatments are increasingly complex and multi-modal and methodological frameworks could also be extended to account for this complexity, identifying dates of the first modality and then subsequent modalities of the overall initial treatment package.
Furthermore, consistent and accurate measurement of time intervals is not easily done; especially, in the initial phase such as in the “appraisal” and “help-seeking” intervals in “The Model of Pathways to Treatment”. While, theoretically, they are distinct stages, it is very difficult to measure them separately. In the IRCO trial and the SYMPTOM-upper gastrointestinal study (19,20), for example, patients found it difficult to distinguish symptom appraisal, reappraisal from the time they decided to seek help. It may be possible to tease these distinctions out through in-depth qualitative interviews, but it is more challenging to capture when using patient surveys. Combining both intervals as the patient interval from symptom onset to first presentation in a clinical setting could be more feasible for consistently measuring across studies. Another challenge in implementing the Model of Pathways to Treatment is that it is not so useful for quantitative research on timeliness.
Proposal of a generalisable framework
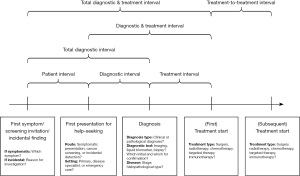
Given the challenges mentioned above, we call for a more generalisable and simplified framework, aiming to better support the clinical research and policy relating to cancer diagnostic and treatment intervals. The main difference of this framework from the two above is including a more generalisable approach with milestones that account for different routes to diagnosis and treatment across a range of healthcare systems (Figure 2). The framework suggests a more feasible approach for quantitative research compared to the Model of Pathways to Treatment, especially the initial phase before patients present in a clinical setting. Also, the framework simplifies the Oleson et al. model to allow research based on visualising and comparing pathways across different health systems (with or without primary care gatekeeper and referral system). Last, the framework adds other routes to diagnosis instead of symptomatic presentation only, as well as considers the timing of first- and subsequent-line treatments. Of note, this proposal only considers the most essential milestones which are generalisable across countries, and does not devalue the importance of further sub-intervals, such as primary care and secondary intervals in countries heavily relying on a gatekeeper and referral system.
In the proposed new framework, we include a brief item list in each milestone, guiding the completeness and the accuracy of reporting times to diagnosis and treatment for rigorous study design and analysis. In addition to the recommended items, we also emphasise the importance of reporting more detailed information if available, including patients’ first and subsequent symptoms and dates of onset, date of screening invitation and date of performing screening test, date of incidental detection and reason for investigation, and type of clinical facility for first and subsequent presentations. For the diagnostic tests performed we recommend, if possible, to provide the details of tests used for initial triage, initial and confirmed diagnosis as well as the molecular characterisation.
Our proposed framework can also inform the development of core datasets using consistent definitions of the main intervals along different diagnostic and treatment pathways. It could guide research on diagnostic and treatment intervals across different healthcare settings, allowing international comparisons to be made and a better understanding of how these contribute to variations in outcomes. The above implications may be applicable for not just cancer but other diseases as well.
Acknowledgments
The work was presented at the Victorian Comprehensive Cancer Centre (VCCC) Alliance Research Conference 2023, on 12–13 September 2023, in Melbourne, Australia.
Funding: The study is supported by an NHMRC Investigator grant (No. APP1195302 to JDE).
Footnote
Provenance and Peer Review: This article was a standard submission to the journal. The article has undergone external peer review.
Peer Review File: Available at https://ace.amegroups.com/article/view/10.21037/ace-23-2/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ace.amegroups.com/article/view/10.21037/ace-23-2/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Global Burden of Disease Cancer Collaboration. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2019;5:1749-68. [Crossref] [PubMed]
- World Health Organization (WHO). GUIDE TO CANCER EARLY DIAGNOSIS. World Health Organization 2017. Available online: https://www.who.int/publications/i/item/9789241511940
- Zhang J, Li J, Xiong S, et al. Global burden of lung cancer: implications from current evidence. Ann Cancer Epidemiol 2021;5:4. [Crossref]
- World Health Organization (WHO). WHO report on cancer: setting priorities, investing wisely and providing care for all. World Health Organization 2020. Available online: https://apps.who.int/iris/handle/10665/330745
- National Institute for Health and Care Excellence (NICE). Suspected Cancer: Recognition and Referral [NG12]. NICE, London 2015. Available online: https://www.nice.org.uk/guidance/ng12
- Nicholson BD, Lyratzopoulos G. Progress and priorities in reducing the time to cancer diagnosis. Br J Cancer 2023;128:468-70. [Crossref] [PubMed]
- Petrova D, Špacírová Z, Fernández-Martínez NF, et al. The patient, diagnostic, and treatment intervals in adult patients with cancer from high- and lower-income countries: A systematic review and meta-analysis. PLoS Med 2022;19:e1004110. [Crossref] [PubMed]
- Zhang J, IJzerman MJ, Oberoi J, et al. Time to diagnosis and treatment of lung cancer: A systematic overview of risk factors, interventions and impact on patient outcomes. Lung Cancer 2022;166:27-39. [Crossref] [PubMed]
- COVIDSurg Collaborative. Effect of COVID-19 pandemic lockdowns on planned cancer surgery for 15 tumour types in 61 countries: an international, prospective, cohort study. Lancet Oncol 2021;22:1507-17. [Crossref] [PubMed]
- Riera R, Bagattini ÂM, Pacheco RL, et al. Delays and Disruptions in Cancer Health Care Due to COVID-19 Pandemic: Systematic Review. JCO Glob Oncol 2021;7:311-23. [Crossref] [PubMed]
- Angelini M, Teglia F, Astolfi L, et al. Decrease of cancer diagnosis during COVID-19 pandemic: a systematic review and meta-analysis. Eur J Epidemiol 2023;38:31-8. [Crossref] [PubMed]
- Zhang L, Carvalho AL, Mosquera I, et al. An international consensus on the essential and desirable criteria for an 'organized' cancer screening programme. BMC Med 2022;20:101. [Crossref] [PubMed]
- Drosdowsky A, Lamb KE, Bergin RJ, et al. A systematic review of methodological considerations in time to diagnosis and treatment in colorectal cancer research. Cancer Epidemiol 2023;83:102323. [Crossref] [PubMed]
- Jacobsen MM, Silverstein SC, Quinn M, et al. Timeliness of access to lung cancer diagnosis and treatment: A scoping literature review. Lung Cancer 2017;112:156-64. [Crossref] [PubMed]
- Weller D, Vedsted P, Rubin G, et al. The Aarhus statement: improving design and reporting of studies on early cancer diagnosis. Br J Cancer 2012;106:1262-7. [Crossref] [PubMed]
- Walter F, Webster A, Scott S, et al. The Andersen Model of Total Patient Delay: a systematic review of its application in cancer diagnosis. J Health Serv Res Policy 2012;17:110-8. [Crossref] [PubMed]
- Olesen F, Hansen RP, Vedsted P. Delay in diagnosis: the experience in Denmark. Br J Cancer 2009;101:S5-8. [Crossref] [PubMed]
- Lynch C, Harrison S, Emery JD, et al. Variation in suspected cancer referral pathways in primary care: comparative analysis across the International Benchmarking Cancer Partnership. Br J Gen Pract 2023;73:e88-94. [Crossref] [PubMed]
- Emery JD, Gray V, Walter FM, et al. The Improving Rural Cancer Outcomes Trial: a cluster-randomised controlled trial of a complex intervention to reduce time to diagnosis in rural cancer patients in Western Australia. Br J Cancer 2018;118:e8. [Crossref] [PubMed]
- Karnchanachari N, Milton S, Muhlen-Schulte T, et al. The SYMPTOM-upper gastrointestinal study: A mixed methods study exploring symptom appraisal and help-seeking in Australian upper gastrointestinal cancer patients. Eur J Cancer Care (Engl) 2022;31:e13605. [Crossref] [PubMed]
Cite this article as: Zhang J, IJzerman MJ, Emery JD. Timely cancer diagnosis and treatment: towards a generalisable research framework studying timeliness to appropriate care. Ann Cancer Epidemiol 2023;7:3.