
Racial/ethnic disparities in HPV-related oropharyngeal cancer outcomes among males in the United States: a national cohort study
Introduction
In 2021, 38,800 new cases and 7,620 deaths from oropharyngeal cancer (OPC) are expected in males in the United States, which is more than double the cases among females (estimated at 15, 210 new cases and 3,230 deaths) (1). OPCs are preventable malignancies that were predominantly linked to tobacco and alcohol use until the 1980s, when incidence declined along with a concomitant decrease in these behaviors (2,3). The resurgence in OPC incidence has been associated with the human papillomavirus (HPV) (4), the most common sexually transmitted infection in the U.S. (5). Males have a threefold higher prevalence of HPV positivity (6,7), are less likely to clear oral HPV infections (8), and have over five times higher OPC incidence rate than females (9). Male HPV-related OPC is now the most prevalent HPV-related cancer in the U.S., exceeding cases of HPV-related cervical cancer (9), rendering male OPC an important new public health concern.
The HPV 9-valent vaccine (Gardasil 9) was approved by the Food and Drug Administration in 2014 in females age 9 through 26 years of age and males age 9 to 15 for the prevention of genital warts and precancerous lesions, and cancers of the cervix, vulvar, vagina, and anus (10). The approval was extended to males age 16 to 26 in 2015, and all adults 27 through 45 years of age in 2018. The indication for prevention of oropharyngeal and other head and neck cancers was added in 2020. The Centers for Disease Control and Prevention (CDC) recommends routine HPV vaccination for all adolescents through age 26 years (11). It advises shared decision-making for adults aged 27 through 45 years who might benefit, such as those not already immune (either through infection or vaccination) and is at risk for future HPV infections (i.e., a new sexual partner). HPV vaccination completion rates among adolescents in 2020 are only 58.6% in the U.S., and significantly lower among males (56.0%) versus females (61.4%) (12). Awareness of HPV and the vaccine has declined recently, with the lowest awareness among males, racial minorities, residents in rural counties, and those of low socioeconomic status (SES) (13).
The CDC estimates 72% of all male OPCs to be HPV-related, specifically squamous cell carcinomas (SCCs) (14). Compared to HPV-negative SCCs, HPV-positive SCCs are more likely in younger persons, with higher SES and education, and lower exposures to tobacco and alcohol (6,15-17). High prevalence of HPV-positive OPC in White males has been previously reported (18), as well as the increasing trends of HPV-positive OPC across all sex and racial/ethnic groups in the U.S.; however, studies did not stratify by stage (18-21). To our knowledge, only one study evaluated racial differences in OPC stage at diagnosis, but it excluded Hispanics and did not differentiate by sex (22). Most studies examining racial differences in OPC survival have been hospital-based (23); studies that were population-based did not include Hispanics (24-28). A recent study that included Hispanic males evaluated stage at diagnosis and survival of head and neck cancers in general, but it only included patients over age 65 years with Medicare fee-for-service insurance (29). Evaluating OPC in Hispanic males is important because they may have limited knowledge of HPV (30). Our study is the largest population-based study to date examining racial/ethnic disparities in incidence, stage at diagnosis, survival, and mortality of HPV-related OPCs among adult males across the U.S. We present this study in accordance with the STROBE reporting checklist (available at https://ace.amegroups.com/article/view/10.21037/ace-22-1/rc).
Methods
Data source and study sample
In this population-based retrospective cohort study, we used the North American Association of Central Cancer Registries (NAACCR) Cancer in North America (CiNA) Research Analytic File to examine males diagnosed between January 1, 2005 to December 31, 2016 with OPC in the U.S. NAACCR CiNA is the most comprehensive cancer incidence database, covering 93% of the U.S. population, and it includes all 18 Surveillance, Epidemiology, and End Results (SEER) registries (31). For survival and mortality analyses, we used a subset of this data file, the CiNA Survival dataset, which includes cancer registries that meet the SEER standards for follow-up or ascertainment of deaths. Data were accessed through the SEER*Stat software program and exported into the Statistical Package for the Social Sciences version 27 (IBM, Armonk, New York, USA) for advanced analyses. All variables analyzed were available in NAACCR CiNA.
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Boards at NAACCR and Rutgers, The State University of New Jersey (Pro2019001220) and determined exempt from review because the research involves secondary analysis of retrospective data that was de-identified.
Because cancer registries do not collect HPV status (32), we used standard CDC definitions of HPV-associated cancers to identify our male cohort: (I) SCC histological codes (8050-8084; 8120-8131); and (II) International Classification of Diseases for Oncology (ICD-O)-3 site codes: base of tongue (C01.9); lingual tonsil (C02.4); overlapping lesion of tongue (C02.8); soft palate (C05.1); uvula (C05.2); tonsillar fossa (C09.0); tonsillar pillar (C09.1); overlapping lesion of tonsil (C09.8); tonsil (C09.9); vallecula (C10.0); anterior surface of epiglottis (C10.1); lateral wall of oropharynx (C10.2); posterior wall of oropharynx (C10.3); branchial cleft (C10.4); overlapping lesion of oropharynx (C10.8); oropharynx (C10.9); pharynx (C14.0); Waldeyer’s ring (C14.2); and overlapping lesion of lip, oral cavity, and pharynx (C14.8). HPV-related OPC were restricted to microscopically confirmed cases (32). These same methods have previously been used by cancer registry-based studies focused on HPV-associated cancers (33,34).
Outcomes
Primary outcomes included incidence, late-stage diagnosis, mean and cumulative survival, and cancer-specific mortality (CSM). Figure 1A depicts how the analytic study samples were constructed for the incidence and late-stage outcomes. We extracted incident cases of male HPV-related OPC from 2005–2016 (N=162,183) from SEER*Stat and calculated yearly age-adjusted rates per 100,000 males by race/ethnicity, stratified by stage. For stage at diagnosis analyses, we excluded persons with unknown stage (N=6,366), race (N=824), and cases with missing covariates (N=21,170). For our logistic regression models, we dichotomized stage into “early” if the OPC was local, and “late” if it was regional or distant (35).
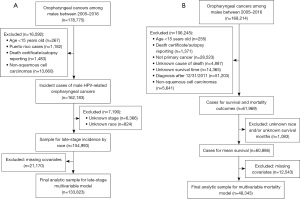
Survival and mortality analyses excluded cases diagnosed after December 31, 2011 to have at least 5 years of follow-up. Figure 1B describes derivation of our analytic study sample for the survival and mortality outcomes. The “SEER cause-specific death classification” variable from the NAACCR CiNA Deluxe data file was used to calculate CSM (36). This variable addresses known misclassifications in cause of death in death certificates for cancer, taking into account cause of death (ICD-10 codes), site of original cancer diagnosis, tumor sequence, and diseases related to the cancer of diagnosis. It categorizes cases as: dead (attributable to this cancer diagnosis); alive or dead of other cause; and dead (missing/unknown cause of death) (37). For CSM, persons who died of causes other than OPC were censored.
Covariates
Our main independent variable was race/ethnicity, categorized as non-Hispanic (NH) White, NH Black, Hispanic, and NH Other (Asian, Pacific Islander, American Indian, and Alaskan Native), as classified in the NAACCR Research File (31). Covariates for late-stage models included: age at diagnosis; health insurance; county level attributes of residence (metropolitan/non-metropolitan, percent of persons below poverty); geographic region of the US. For our mortality models, stage at diagnosis and treatment modality were also included. The NAACCR CiNA Research file categorizes age in 5-year intervals starting at age 15 (e.g., 35–39, 40–44). We grouped age into three categories: <55, 55–64, 65+ years based on distribution and age of Medicare eligibility. Health insurance categories included: private, Medicare, Medicaid, other (Indian/Public Health Service, Military, TRICARE, Veterans Affairs, and insurance not otherwise specified), and no insurance/self-pay. Metropolitan/non-metropolitan county designations were broadly based on population size (metropolitan has 50,000 persons or more) (38). Percent of persons below poverty level in the county was categorized as: ≤9.99%, 10–19.99%, and 20% or more below federal poverty levels. The four geographic regions of the U.S. were based on the U.S. Census Bureau: Northeast, South, Midwest, and West/Pacific (39). Treatment modality included the first course of planned treatment and included the following categories: surgery only; radiation or chemotherapy only; surgery plus (radiation or chemotherapy); radiation and chemotherapy; all modalities; or no treatment.
Statistical analysis
Bivariate relationships between race/ethnicity and demographics, stage, and treatment were evaluated using chi-square tests. Adjusted odds ratios (aOR) of late-stage diagnosis compared with early stage and corresponding 95% confidence intervals (CIs) were calculated for each category of race/ethnicity using multivariable logistic regression. Mean cancer-specific survival for each racial/ethnic group was calculated within SEER*Stat using the actuarial method via the SEER cause-specific death classification variable. Adjusted cancer-specific survival curves for each racial/ethnic group were compared using Cox proportional hazards regression. We examined associations of race/ethnicity with mortality from OPC using Cox proportional hazards regression models to estimate adjusted hazard ratios (aHR) and 95% CIs. We included covariates described above and stage at diagnosis in multivariable Model 1. To determine the effect of treatment modality on the associations between race/ethnicity and mortality, we added initial treatment modality to Model 2 to see how hazards ratios observed in Model 1 changed. Finally, interaction terms of race/ethnicity with all other covariates were tested in the final late-stage and mortality models.
We conducted sensitivity analyses including and excluding unknown race/ethnicity and unknown covariates. Results were similar; therefore, we present multivariable models excluding missing values. Final analytic sample sizes were 133,823 for late-stage and 48,343 for CSM multivariable models (see Table S1). All statistical tests were 2-sided; P<0.05 was considered significant.
Results
Table 1 describes characteristics of our cohort, stratified by race/ethnicity. The highest proportion of cases were in NH Whites (84.2%), age 55–64 years (38.7%), with private insurance (33.7%), from metropolitan counties (82%), in the geographic South (40.2%), and counties with 10–19.99% of persons below poverty (68.5%). Most cases were diagnosed at regional stage (66.1%). Compared to other racial/ethnic groups, a higher proportion of NH Black males had Medicaid insurance (16.9%), lived in the South (54.8%), in counties with ≥20% poverty (32.6%), and had distant stage at diagnosis (25.1%). Additionally, a higher percentage of Hispanic (13.1%) and NH Black males (13.8%) received no treatment (all P values <0.01).
Table 1
| Characteristic† | Total, N (%) | Hispanic, N (%) | NH White, N (%) | NH Black, N (%) | NH Other‡, N (%) | Unknown, N (%) |
|---|---|---|---|---|---|---|
| Overall | 162,183 (100.0) | 7,825 (4.8) | 136,537 (84.2) | 14,283 (8.8) | 2,714 (1.7) | 824 (0.5) |
| Age at diagnosis, years | ||||||
| <55 | 46,116 (28.4) | 2,421 (30.9) | 38,343 (28.1) | 4,294 (30.1) | 803 (29.6) | 255 (30.9) |
| 55–64 | 62,813 (38.7) | 2,811 (35.9) | 53,090 (38.9) | 5,623 (39.4) | 965 (35.6) | 324 (39.3) |
| 65+ | 53,254 (32.8) | 2,593 (33.1) | 45,104 (33.0) | 4,366 (30.6) | 946 (34.9) | 245 (29.7) |
| County of residence | ||||||
| Metropolitan | 132,965 (82.0) | 7,345 (93.9) | 110,216 (80.7) | 12,460 (87.2) | 2,259 (83.2) | 685 (83.1) |
| Non-metropolitan | 26,656 (16.4) | 451 (5.8) | 23,949 (17.5) | 1,751 (12.3) | 408 (15.0) | 97 (11.8) |
| Unknown | 2,562 (1.6) | 29 (0.4) | 2,372 (1.7) | 72 (0.5) | 47 (1.7) | 42 (5.1) |
| Insurance status | ||||||
| Private | 54,633 (33.7) | 2,187 (27.9) | 48,557 (35.6) | 2,789 (19.5) | 875 (32.2) | 225 (27.3) |
| Medicare | 44,925 (27.7) | 1,878 (24.0) | 38,226 (28.0) | 4,034 (28.2) | 657 (24.2) | 130 (15.8) |
| Medicaid | 12,473 (7.7) | 954 (12.2) | 8,790 (6.4) | 2,407 (16.9) | 279 (10.3) | 43 (5.2) |
| Other§ | 19,677 (12.1) | 719 (9.2) | 16,736 (12.3) | 1,726 (12.1) | 354 (13.0) | 142 (17.2) |
| No insurance/self pay | 7,133 (4.4) | 605 (7.7) | 5,357 (3.9) | 1,013 (7.1) | 123 (4.5) | 35 (4.2) |
| Unknown | 23,342 (14.4) | 1,482 (18.9) | 18,871 (13.8) | 2,314 (16.2) | 426 (15.7) | 249 (30.2) |
| % persons below poverty at county of residence | ||||||
| ≤9.9% | 26,530 (16.4) | 715 (9.1) | 23,933 (17.5) | 1,102 (7.7) | 658 (24.2) | 122 (14.8) |
| 10–19.99% | 111,047 (68.5) | 5,828 (74.5) | 94,480 (69.2) | 8,450 (59.2) | 1,689 (62.2) | 600 (72.8) |
| ≥20% | 22,044 (13.6) | 1,253 (16.0) | 15,752 (11.5) | 4,659 (32.6) | 320 (11.8) | 60 (7.3) |
| Unknown | 2,562 (1.6) | 29 (0.4) | 2,372 (1.7) | 72 (0.5) | 47 (1.7) | 42 (5.1) |
| Geographic region | ||||||
| Northeast | 27,741 (17.1) | 1,517 (19.4) | 23,637 (17.3) | 2,143 (15.0) | 352 (13.0) | 92 (11.2) |
| South | 65,133 (40.2) | 3,120 (39.9) | 53,269 (39.0) | 7,834 (54.8) | 650 (23.9) | 260 (31.6) |
| Midwest | 36,003 (22.2) | 568 (7.3) | 31,852 (23.3) | 3,087 (21.6) | 333 (12.3) | 163 (19.8) |
| West/Pacific | 33,306 (20.5) | 2,620 (33.5) | 27,779 (20.3) | 1,219 (8.5) | 1,379 (50.8) | 309 (37.5) |
| Stage at diagnosis | ||||||
| Local | 20,420 (12.6) | 953 (12.2) | 17,172 (12.6) | 1,811 (12.7) | 357 (13.2) | 127 (15.4) |
| Regional | 107,170 (66.1) | 4,924 (62.9) | 91,794 (67.2) | 8,332 (58.3) | 1,692 (62.3) | 428 (51.9) |
| Distant | 28,227 (17.4) | 1,511 (19.3) | 22,497 (16.5) | 3,585 (25.1) | 530 (19.5) | 104 (12.6) |
| Unknown | 6,366 (3.9) | 437 (5.6) | 5,074 (3.7) | 555 (3.9) | 135 (5.0) | 165 (20.0) |
| Treatment¶ | ||||||
| Surgery only | 14,668 (9.0) | 748 (9.6) | 12,444 (9.1) | 1,126 (7.9) | 250 (9.2) | 100 (12.2) |
| Radiation or chemotherapy | 19,743 (12.2) | 1,031 (13.2) | 15,998 (11.7) | 2,350 (16.5) | 286 (10.5) | 78 (9.5) |
| Surgery + (radiation or chemotherapy) | 37,409 (23.1) | 1,533 (19.6) | 32,891 (24.1) | 2,241 (15.7) | 604 (22.3) | 140 (17.0) |
| Radiation + chemotherapy | 50,485 (31.1) | 2,425 (31.0) | 41,816 (30.6) | 5,189 (36.3) | 847 (31.2) | 208 (25.3) |
| All modalities | 23,933 (14.8) | 1,061 (13.6) | 20,950 (15.3) | 1,407 (9.9) | 438 (16.1) | 77 (9.4) |
| No treatment | 15,905 (9.8) | 1,025 (13.1) | 12,404 (9.1) | 1,967 (13.8) | 289 (10.6) | 220 (26.7) |
†, all variables were statistically significant at P<0.01 in bivariate analyses using χ2 tests; ‡, includes Asian, American Indian, Alaska Native, and Pacific Islander; §, includes Indian/Public Health Service, Military, TRICARE, Veterans Affairs, and insurance not otherwise specified; ¶, cases with unknown treatment are not shown due to cells having counts less than 6. HPV, human papillomavirus; NH, non-Hispanic.
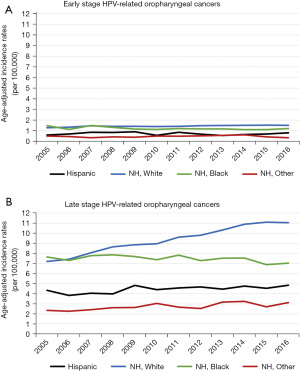
From 2005–2016, the age-adjusted incidence rates of HPV-related early-stage OPCs remained stable among all race/ethnicities (Figure 2A). However, late-stage OPCs increased steadily among NH White males, while remaining relatively stable in the other racial/ethnic groups (Figure 2B).
Stage at diagnosis
Table 2 presents independent factors associated with late-stage OPC diagnosis. No significant difference in late-stage diagnosis existed between NH White, NH Black, and Hispanics, after adjusting for age, insurance, county level attributes of residence (Metropolitan/Non-metropolitan, percent of persons below poverty), and geographic region. NH other males had lower odds (aOR 0.87, 95% CI: 0.77–0.99) of late-stage diagnosis relative to NH Whites. Predictors of late-stage diagnosis included having Medicaid (aOR, 1.32; 95% CI: 1.23–1.42) or no insurance (aOR, 1.40; 95% CI: 1.28–1.53). Factors associated with lower odds of late-stage diagnosis included age greater than 55 years, having Medicare or other insurance (relative to private insurance), residence in non-metropolitan county, and the South or Midwest regions (relative to the Northeast). There were no significant interactions between race/ethnicity and other variables.
Table 2
| Characteristic | aOR‡ (95% CI) |
|---|---|
| Race/ethnicity | |
| Non-Hispanic White | 1.00 |
| Hispanic/Latino | 0.94 (0.87, 1.02) |
| Non-Hispanic Black | 0.99 (0.94, 1.06) |
| Non-Hispanic other§ | 0.87 (0.77, 0.99)* |
| Age at diagnosis (years) | |
| <55 | 1.00 |
| 55–64 | 0.91 (0.87, 0.95)* |
| 65+ | 0.68 (0.65, 0.72)* |
| Insurance type | |
| Private | 1.00 |
| Medicare | 0.81 (0.77, 0.85)* |
| Medicaid | 1.32 (1.23, 1.42)* |
| Other¶ | 0.94 (0.89, 0.99)* |
| No insurance/self pay | 1.40 (1.28, 1.53)* |
| County of residence | |
| Large metropolitan | 1.00 |
| Non-metropolitan | 0.94 (0.90, 0.98)* |
| % persons below poverty at county of residence | |
| ≤9.9% | 1.00 |
| 10–19.99% | 0.97 (0.93, 1.02) |
| ≥20% | 0.95 (0.89, 1.01) |
| Geographic region of U.S. | |
| Northeast | 1.00 |
| South | 0.84 (0.70, 0.89)* |
| Midwest | 0.92 (0.87, 0.98)* |
| West/Pacific | 1.06 (0.99, 1.12) |
†, cases with unknown stage and unknown covariates were excluded; ‡, adjusted for all other variables in table; §, includes Asian, American Indian, Alaska Native, and Pacific Islander; ¶, includes Indian/Public Health Service, Military, TRICARE, Veterans Affairs, and insurance not otherwise specified; *, indicate significance at P≤0.01. HPV, human papillomavirus; aOR, adjusted odds ratio; CI, confidence interval.
Survival and mortality
Among the 61,969 males diagnosed with HPV related OPC from 2005–2011, there were 14,771 deaths from OPC during the follow-up period (at least 5 years). NH Black and Hispanic males had lower unadjusted mean survival times compared to NH White males, with NH Black males having 30 months shorter survival (P<0.01) (Table 3). Figure 3 depicts the adjusted cumulative survival curves by race/ethnicity of male HPV-related OPCs. NH Black males had the lowest cumulative survival relative to the other racial/ethnic groups; by 5 years after diagnosis, only 60% survived versus over 70% for the other groups (P<0.001).
Table 3
| Change to race/ethnicity | Mean (95% CI), months |
|---|---|
| Overall | 96.58 (96.16–97.00) |
| Hispanic | 91.89 (89.87–93.91) |
| NH White | 99.63 (99.18–100.07) |
| NH Black | 69.72 (68.14–71.31) |
| NH other‡ | 96.55 (93.25–99.84) |
†, unadjusted; All log rank Mantel-Cox tests were statistically significant at P<0.01; ‡, includes Asian, American Indian, Alaska Native, and Pacific Islander. HPV, human papillomavirus; NH, non-Hispanic.
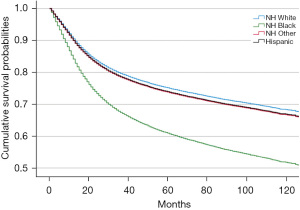
Table 4 describes independent predictors of CSM. In multivariable analyses adjusting for all covariates except treatment modality (Model 1), NH Black and Hispanic males, age over 54 years, all insurances other than private, residence in counties with higher poverty, and living in the Northeast were associated with higher mortality. Adjusting further for treatment modality (Model 2) attenuated, but did not eliminate, the higher mortality in Hispanic (aHR 1.10; 95% CI: 1.02–1.18) and NH Black (aHR 1.74; 95% CI: 1.66–1.83) males. Compared with having surgery only, receipt of radiation or chemotherapy only was associated with higher mortality (aHR 1.86; 95% CI: 1.71–2.01), and no treatment conferred almost 4-fold higher mortality (aHR 3.73, 95% CI: 3.41–4.08).
Table 4
| Characteristic | Model 1‡, aHR (95% CI) | Model 2§, aHR (95% CI) |
|---|---|---|
| Race/ethnicity | ||
| Non-Hispanic, White | 1.00 | 1.00 |
| Hispanic/Latino | 1.17 (1.08, 1.26)* | 1.10 (1.02, 1.18)* |
| Non-Hispanic, Black | 1.79 (1.71, 1.88)* | 1.74 (1.66, 1.83)* |
| Non-Hispanic, other¶ | 1.06 (0.93, 1.20) | 1.04 (0.91, 1.19) |
| Age at diagnosis (years) | ||
| <55 | 1.00 | 1.00 |
| 55–64 | 1.22 (1.18, 1.27)* | 1.16 (1.11, 1.20)* |
| 65+ | 1.63 (1.54, 1.72)* | 1.48 (1.40, 1.57)* |
| Insurance type | ||
| Private | 1.00 | 1.00 |
| Medicare | 1.94 (1.84, 2.05)* | 1.85 (1.75, 1.95)* |
| Medicaid | 2.95 (2.80, 3.11)* | 2.70 (2.56, 2.85)* |
| Other†† | 1.45 (1.38, 1.53)* | 1.40 (1.33, 1.48)* |
| No insurance/self pay | 2.40 (2.25, 2.56)* | 2.10 (1.97, 2.24)* |
| Stage at diagnosis | ||
| Local | 1.00 | 1.00 |
| Regional | 1.32 (1.24, 1.40)* | 1.39 (1.31, 1.49)* |
| Distant | 3.08 (2.89, 3.28)* | 3.14 (2.94, 3.37)* |
| County of residence | ||
| Large metropolitan | 1.00 | 1.00 |
| Non-metropolitan | 0.99 (0.95, 1.04) | 1.00 (0.96, 1.05) |
| % persons below poverty at county of residence | ||
| ≤9.9% | 1.00 | 1.00 |
| 10–19.99% | 1.15 (1.09, 1.21)* | 1.13 (1.07, 1.19)* |
| ≥20% | 1.28 (1.20, 1.37)* | 1.25 (1.17, 1.33)* |
| Geographic region of U.S. | ||
| Northeast | 1.00 | 1.00 |
| South | 1.03 (0.97, 1.08) | 1.00 (0.94, 1.06) |
| Midwest | 0.81 (0.77, 0.87)* | 0.86 (0.81, 0.92)* |
| West/Pacific | 0.91 (0.85, 0.96)* | 0.93 (0.87, 0.99)* |
| Treatment modality | ||
| Surgery only | 1.00 | |
| Radiation or chemotherapy | 1.86 (1.71, 2.01)* | |
| Surgery + (radiation or chemotherapy) | 0.82 (0.74, 0.91)* | |
| Radiation + chemotherapy | 1.19 (1.10, 1.28)* | |
| All modalities | 0.77 (0.71, 0.84)* | |
| No treatment | 3.73 (3.41, 4.08)* |
†, excluded cases diagnosed after December 31, 2011, if not first primary cancer, had unknown cause of death, unknown survival time, or missing covariates; ‡, adjusted for all other variables except treatment modality; §, adjusted for all other variables including treatment modality; ¶, includes American Indian, Alaska Native, Asian, and Pacific Islander; ††, includes Indian/Public Health Service, Military, TRICARE, Veterans Affairs, and insurance not otherwise specified; *, indicate significance at P≤0.01. aHR, adjusted hazards ratio; CI, confidence interval.
Race modified the associations of mortality with age, insurance, stage, and region (Table 5). For example, compared to NH White males of the same age group, NH Black males less than age 55 years had almost 3-fold higher CSM (aHR 2.97; 95% CI: 2.78–3.18), while NH Black males aged 55–64 and 65 years or older had lower mortality (aHR 0.89; 95% CI: 0.80–0.99 and aHR 0.75; 95% CI: 0.65–0.87, respectively). Conversely, Hispanic males of all age groups had higher mortality, with higher hazards of death with each successive age group when compared with NH White males of the same age group. Additionally, compared with NH White males at the same stage at diagnosis, Hispanic males with local stage had higher mortality (aHR 1.30; 95% CI: 1.04–1.62), while those with regional or distant stage had similar mortality. Furthermore, NH Black males in the Northeast had over 2 times higher mortality (aHR 2.33; 95% CI: 2.03–2.66) relative to White males in the Northeast, while those living in the Midwest or West/Pacific had lower mortality (aHR 0.82; 95% CI: 0.67–0.98 and aHR 0.74; 95% CI: 0.60–0.92, respectively).
Table 5
| Characteristic | aHR‡ (95% CI) | P value |
|---|---|---|
| Age (years) × race/ethnicity | <0.01* | |
| <55 | ||
| White | 1.00 | |
| Hispanic | 1.16 (1.03, 1.31) | 0.02* |
| NH, Black | 2.97 (2.78, 3.18) | <0.01* |
| NH, other§ | 1.18 (0.96, 1.45) | 0.11 |
| 55–64 | ||
| White | 1.00 | |
| Hispanic | 1.21 (1.01, 1.46) | 0.04* |
| NH, Black | 0.89 (0.80, 0.99) | 0.04* |
| NH, other§ | 1.08 (0.79, 1.48) | 0.63 |
| 65+ | ||
| White | 1.00 | |
| Hispanic | 1.30 (1.03, 1.64) | 0.03* |
| NH, Black | 0.75 (0.65, 0.87) | <0.01* |
| NH, other§ | 0.58 (0.39, 0.88) | 0.01 |
| Insurance × race/ethnicity | <0.01* | |
| Private | ||
| White | 1.00 | |
| Hispanic | 1.13 (0.96, 1.32) | 0.14 |
| NH, Black | 2.25 (2.03, 2.51) | <0.01* |
| NH, other§ | 1.10 (0.86, 1.41) | 0.45 |
| Medicare | ||
| White | 1.00 | |
| Hispanic | 1.16 (0.91, 1.49) | 0.23 |
| NH, Black | 0.97 (0.83, 1.13) | 0.68 |
| NH, other§ | 1.40 (0.91, 2.14) | 0.12 |
| Medicaid | ||
| White | 1.00 | |
| Hispanic | 0.96 (0.77, 1.21) | 0.74 |
| NH, Black | 0.74 (0.64, 0.86) | <0.01* |
| NH, other§ | 0.91 (0.59, 1.39) | 0.65 |
| Other¶ | ||
| White | 1.00 | |
| Hispanic | 1.08 (0.82, 1.42) | 0.58 |
| NH, Black | 0.99 (0.84, 1.16) | 0.90 |
| NH, other§ | 1.25 (0.85, 1.82) | 0.26 |
| No insurance/self pay | ||
| White | 1.00 | |
| Hispanic | 1.04 (0.80, 1.36) | 0.75 |
| NH, Black | 0.84 (0.70, 1.00) | 0.04* |
| NH, other§ | 1.03 (0.61, 1.73) | 0.93 |
| Stage × race/ethnicity | <0.01* | |
| Local | ||
| White | 1.00 | |
| Hispanic | 1.30 (1.04, 1.62) | 0.02* |
| NH, Black | 1.80 (1.53, 2.11) | <0.01* |
| NH, other§ | 0.68 (0.41, 1.11) | 0.12 |
| Regional | ||
| White | 1.00 | |
| Hispanic | 0.95 (0.73, 1.23) | 0.69 |
| NH, Black | 1.55 (1.28, 1.87) | <0.01* |
| NH, other§ | 1.23 (0.72, 2.12) | 0.45 |
| Distant | ||
| White | 1.00 | |
| Hispanic | 0.85 (0.65, 1.12) | 0.24 |
| NH, Black | 1.20 (0.98, 1.45) | 0.06 |
| NH, other§ | 0.98 (0.56, 1.72) | 0.96 |
| Geographic region × race/ethnicity | <0.01* | |
| Northeast | ||
| White | 1.00 | |
| Hispanic | 0.89 (0.71, 1.12) | 0.33 |
| NH, Black | 2.33 (2.03, 2.66) | <0.01* |
| NH, other§ | 0.63 (0.37, 1.09) | 0.10 |
| South | ||
| White | 1.00 | |
| Hispanic | 0.93 (0.71, 1.22) | 0.62 |
| NH, Black | 1.05 (0.89, 1.23) | 0.57 |
| NH, other§ | 1.05 (0.56, 1.97) | 0.88 |
| Midwest | ||
| White | 1.00 | |
| Hispanic | 0.67 (0.45, 1.00) | 0.05* |
| NH, Black | 0.82 (0.67, 0.98) | 0.03* |
| NH, other§ | 0.77 (0.37, 1.62) | 0.49 |
| West/Pacific | ||
| White | 1.00 | |
| Hispanic | 1.14 (0.87, 1.48) | 0.35 |
| NH, Black | 0.74 (0.60, 0.92) | 0.01* |
| NH, other§ | 0.94 (0.52, 1.72) | 0.85 |
†, excluded cases diagnosed after December 31, 2011, if not first primary cancer, had unknown cause of death, unknown survival time, or missing covariates; ‡, adjusted for race, age, insurance, HPV status, stage, county of residence, % persons below poverty in county, geographic region, treatment modality; §, includes American Indian, Alaska Native, Asian, and Pacific Islander; ¶, includes Indian/Public Health Service, Military, TRICARE, Veterans Affairs, and insurance not otherwise specified; *, indicate significance at P≤0.05. aHR, adjusted hazards ratio; CI, confidence interval; NH, non-Hispanic; HPV, human papillomavirus.
Discussion
To our knowledge this is the largest and most comprehensive population-based study to date examining relationships between race/ethnicity and incidence, late-stage diagnosis, survival, and mortality among males with HPV-related OPC across the U.S. A majority of cases were diagnosed at regional or distant stage (Table 1). Potential reasons for the late-stage diagnoses include the asymptomatic nature of disease progression (40), resulting in longer delays in diagnosis (41,42). Similar to other studies, NH White males comprised the largest proportion of OPCs (18). While incidence rates of early and late-stage HPV-related OPC remained stable for other racial/ethnic groups, incidence of late-stage HPV-related OPC among NH White males increased over 50% from 2005 to 2016 (Figure 2). Previous studies have also found NH White males representing a large proportion of HPV-related OPC (21,43), which may be related to differences in risk factors for acquiring oral HPV infections, notably oral sexual behavior (44). NH White males have been reported to engage in oral sex at younger ages and have greater number of lifetime sexual partners relative to NH Black males (45).
Although NH Black males had similar odds of late-stage diagnosis compared to NH White males (Table 2), they had significantly lower mean (Table 3) and cumulative survival (Figure 3). This demographic group also had higher mortality, that was not explained by stage or treatment. Contributors to this disparity may include lower SES, limited access to care, receiving care at lower quality hospitals, and being referred for surgical treatment less frequently when compared with other racial/ethnic groups (29,46-49). However, our mortality models adjusted for residence in poverty areas, insurance, stage, and treatment modality. Our results differ from McDermott et al., who found the higher mortality among NH Black patients with head and neck cancer was eliminated after adjusting for stage and treatment (29). Their study focused on patients over 65 years of age with Medicare insurance and included women and cancers other than OPC. Although not captured in the NAACCR database, one factor contributing to the disparity in survival among NH Black males of our cohort may be tobacco use (50), another risk factor for OPC. NH Black males have higher prevalence and longer duration of smoking and lower rates of smoking cessation when compared to NH White males (51,52).
One explanation for the higher mortality in NH Black males younger than 55 years may be the co-infection of HPV and human immunodeficiency virus (HIV) (53). Young NH Black males make up a disproportionate number of new HIV cases (54), carry the largest burden of disease across different racial/ethnic groups (particularly in the South) (55), and are more likely to progress to advanced immunosuppression (56). OPC attributable to HPV have been observed to commonly affect immunosuppressed patients (57), and HPV increases the incidence of OPC threefold in HIV positive individuals compared to the general population (58).
We found no differences between Hispanic and NH White males in late-stage diagnosis after adjusting for covariates (Table 2). However, Hispanic males overall, and particularly those diagnosed at local stage, had higher CSM relative to NH White males (Table 5). This observation may also be partially explained by the HIV epidemic among Hispanic and Latinx males (59). Additionally, NH other males had lower odds of late-stage disease compared with NH White males (Table 2), with no significant interactions observed in our stage or mortality models. We are the first to report HPV-related OPC outcomes among Hispanic and NH other males of all ages and of different subgroups nationwide. The majority of previous literature predominantly focused on understanding disease burden between sexes (8,18,43), NH White-Black disparities (21,22,24,50,60), or NH Black-Hispanic differences among elderly Medicare recipients with head and neck cancers (29). Since there is considerable intragroup variability among Hispanic (61) and Asian populations (62), future studies are needed to understand HPV-related OPC outcomes among these ethnicities and their subgroups.
Having Medicaid or no insurance were independent predictors of late-stage diagnosis (Table 2) with over 2-fold increase in mortality compared with private insurance (Table 4), which may be related to lower access to dental care and receipt of intra- or extra-oral cancer screening exams (63). Even among patients with dental insurance, studies have identified lack of provider health literacy surrounding HPV-related cancers as a potential barrier in performing oral cancer screenings and providing education on HPV vaccination (64). These findings magnify the importance of primary care providers in prevention and early detection of OPCs among patients with limited access to dental care, such as those with Medicaid or no insurance coverage. However, Hurley et al. reported knowledge gaps among primary care physicians in terms of the most recent HPV vaccine recommendations, vaccine efficacy by age groups, and overall disease progression of HPV cancers or infections (65).
Several potential limitations should be considered when interpreting our results. First, our study was limited to data within the NAACCR database, which omits important information, particularly health behaviors (e.g., smoking and alcohol), comorbid conditions (e.g., HIV), sexual practices, and usual source of care, which may influence OPC incidence, stage at diagnosis, and mortality. For example, we were unable to control for the potential confounding effects of smoking when comparing OPC survival by race/ethnicity. Prior research has shown that both HPV infections and smoking status are known to vary by race/ethnicity (66), and recent data show that NH Black males continue to have higher smoking rates while Hispanic males have lower smoking rates compared with NH Whites (52). Additionally, we did not have the ability to molecularly confirm HPV status since it is not available in the NAACCR database. This may have led to an overestimate of the number of OPC SCCs in our cohort and biased our findings upward from the null (32). Further research is needed to confirm our findings using molecular HPV data and smoking behavior to disentangle the relationship between race/ethnicity, HPV status, and OPC survival. Nevertheless, the NAACCR datafile is the largest cancer incidence database covering 93% of the U.S. population, and this study provides interesting insights, particularly for Hispanic and NH other males, who are generally excluded from studies like this due to small sample size. A final limitation is that the NAACCR data lacks individual level SES data, which may have different impacts on OPC outcomes.
Our findings highlight racial/ethnic disparities in OPC among males, with increasing age-adjusted incidence of late-stage HPV-related OPC in NH White males, and NH Black males having lowest survival and highest CSM, regardless of stage and treatment. Higher CSM was additionally observed in Hispanic males relative to NH White males, particularly those diagnosed at local stage. When considering the strong association between HPV and OPC (4), as well as the cancer preventive potential of vaccination (67), our results call for increasing HPV immunization among all eligible males. One of the unintended consequences of the initial implementation of the HPV vaccine was the “feminization” of this sexually transmitted infection (STI) and its link to cervical cancer (68). This failed to effectively market the additional association of HPV with OPC, setting the stage for the shift in incidence of HPV-related OPC to exceed HPV-related cervical cancers (6). Campaigns should be aimed at increasing awareness of HPV and its link to OPC, as well as increasing vaccine completion in adolescent and young adult males. Additionally, although there are no validated screening tools presently recommended for HPV-related OPC, increasing awareness of primary care providers and dental professionals of risk factors and clinical presentations of OPC, as well as improving their skills in detecting early OPC lesions in patients presenting with symptoms are crucial to decrease late-stage diagnosis (69). Furthermore, access to treatment is paramount to decrease mortality of HPV-related OPC among males. Finally, future studies are needed to elucidate reasons for worse OPC outcomes in NH Black as well as certain subgroups of Hispanic males, such as health behaviors, comorbidities, structural factors (e.g., access to care), medical mistrust, or other vulnerabilities due to lived experiences of bias and racism.
Acknowledgments
The data underlying this article were provided by The North American Association of Central Cancer Registries, which granted access to the Cancer in North America Deluxe file. We thank Anna Petrova, MD, PhD, MPH and Ambarina Faiz, MD, PhD for their feedback on this project as part of Seiichi Villalona’s Distinction in Research Program at Rutgers Robert Wood Johnson Medical School. A previous version of the abstract was published online on May 28, 2021 as part of the 2021 American Society of Clinical Oncology annual meeting and also presented at the 2021 North American Primary Care Research Group Annual Meeting in November 2021. (Top 10 Distinguished Papers).
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://ace.amegroups.com/article/view/10.21037/ace-22-1/rc
Peer Review File: Available at https://ace.amegroups.com/article/view/10.21037/ace-22-1/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ace.amegroups.com/article/view/10.21037/ace-22-1/coif). AMS received travel expenses paid by the North American Association of Central Cancer Registries (NAACCR) while serving on Board of Directors from 2009-2020. Travel reimbursed planned by NAACCR, serving as Co-Chair of NAACCR Communications Steering Committee (2022). The other authors have no conflicts of interests to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Boards at NAACCR and Rutgers, The State University of New Jersey (Pro2019001220) and determined exempt from review because the research involves secondary analysis of retrospective data that was de-identified.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Blot WJ, McLaughlin JK, Winn DM, et al. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res 1988;48:3282-7. [PubMed]
- Montero PH, Patel PD, Palmer FL, et al. Changing trends in smoking and alcohol consumption in patients with oral cancer treated at Memorial Sloan-Kettering Cancer Center from 1985 to 2009. Arch Otolaryngol Head Neck Surg 2012;138:817-22. [Crossref] [PubMed]
- Mourad M, Jetmore T, Jategaonkar AA, et al. Epidemiological Trends of Head and Neck Cancer in the United States: A SEER Population Study. J Oral Maxillofac Surg 2017;75:2562-72. [Crossref] [PubMed]
- Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2018: Human Papillomavirus. Available online: https://www.cdc.gov/std/hpv/stats.htm
- Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 2011;29:4294-301. [Crossref] [PubMed]
- Elrefaey S, Massaro MA, Chiocca S, et al. HPV in oropharyngeal cancer: the basics to know in clinical practice. Acta Otorhinolaryngol Ital 2014;34:299-309. [PubMed]
- D'Souza G, Wentz A, Kluz N, et al. Sex Differences in Risk Factors and Natural History of Oral Human Papillomavirus Infection. J Infect Dis 2016;213:1893-6. [Crossref] [PubMed]
- Centers for Disease Control and Prevention. Cancers Associated with Human Papillomavirus, United States-- 2013-2017. USCS Data Brief, no 18. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2020.
- U.S. Food & Drug Administration. Gardasil 9: United States Government; 2020. Available online: https://www.fda.gov/vaccines-blood-biologics/vaccines/gardasil-9
- Meites E, Szilagyi PG, Chesson HW, et al. Human Papillomavirus Vaccination for Adults: Updated Recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep 2019;68:698-702. [Crossref] [PubMed]
- Pingali C, Yankey D, Elam-Evans LD, et al. National, Regional, State, and Selected Local Area Vaccination Coverage Among Adolescents Aged 13-17 Years - United States, 2020. MMWR Morb Mortal Wkly Rep 2021;70:1183-90. [Crossref] [PubMed]
- Chido-Amajuoyi OG, Jackson I, Yu R, et al. Declining awareness of HPV and HPV vaccine within the general US population. Hum Vaccin Immunother 2021;17:420-7. [Crossref] [PubMed]
- Viens LJ, Henley SJ, Watson M, et al. Human Papillomavirus-Associated Cancers - United States, 2008-2012. MMWR Morb Mortal Wkly Rep 2016;65:661-6. [Crossref] [PubMed]
- D'Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med 2007;356:1944-56. [Crossref] [PubMed]
- Benard VB, Johnson CJ, Thompson TD, et al. Examining the association between socioeconomic status and potential human papillomavirus-associated cancers. Cancer. 2008;113:2910-8. [Crossref] [PubMed]
- Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010;363:24-35. [Crossref] [PubMed]
- Faraji F, Rettig EM, Tsai HL, et al. The prevalence of human papillomavirus in oropharyngeal cancer is increasing regardless of sex or race, and the influence of sex and race on survival is modified by human papillomavirus tumor status. Cancer 2019;125:761-9. [Crossref] [PubMed]
- D'Souza G, Westra WH, Wang SJ, et al. Differences in the Prevalence of Human Papillomavirus (HPV) in Head and Neck Squamous Cell Cancers by Sex, Race, Anatomic Tumor Site, and HPV Detection Method. JAMA Oncol 2017;3:169-77. [Crossref] [PubMed]
- Fakhry C, Cohen E. The rise of HPV-positive oropharyngeal cancers in the United States. Cancer Prev Res (Phila) 2015;8:9-11. [Crossref] [PubMed]
- Zandberg DP, Liu S, Goloubeva OG, et al. Emergence of HPV16-positive oropharyngeal cancer in Black patients over time: University of Maryland 1992-2007. Cancer Prev Res (Phila) 2015;8:12-9. [Crossref] [PubMed]
- Megwalu UC, Ma Y. Racial Disparities in Oropharyngeal Cancer Stage at Diagnosis. Anticancer Res 2017;37:835-9. [Crossref] [PubMed]
- Stein E, Lenze NR, Yarbrough WG, et al. Systematic review and meta-analysis of racial survival disparities among oropharyngeal cancer cases by HPV status. Head Neck 2020;42:2985-3001. [Crossref] [PubMed]
- Osazuwa-Peters N, Massa ST, Christopher KM, et al. Race and sex disparities in long-term survival of oral and oropharyngeal cancer in the United States. J Cancer Res Clin Oncol 2016;142:521-8. [Crossref] [PubMed]
- Razzaghi H, Saraiya M, Thompson TD, et al. Five-year relative survival for human papillomavirus-associated cancer sites. Cancer 2018;124:203-11. [Crossref] [PubMed]
- Brown LM, Check DP, Devesa SS. Oropharyngeal cancer incidence trends: diminishing racial disparities. Cancer Causes Control 2011;22:753-63. [Crossref] [PubMed]
- Goodman MT, Saraiya M, Thompson TD, et al. Human papillomavirus genotype and oropharynx cancer survival in the United States of America. Eur J Cancer 2015;51:2759-67. [Crossref] [PubMed]
- Saba NF, Goodman M, Ward K, et al. Gender and ethnic disparities in incidence and survival of squamous cell carcinoma of the oral tongue, base of tongue, and tonsils: a surveillance, epidemiology and end results program-based analysis. Oncology 2011;81:12-20. [Crossref] [PubMed]
- McDermott JD, Eguchi M, Morgan R, et al. Elderly Black Non-Hispanic Patients With Head and Neck Squamous Cell Cancer Have the Worst Survival Outcomes. J Natl Compr Canc Netw 2020;19:57-67. [Crossref] [PubMed]
- Adjei Boakye E, Tobo BB, Rojek RP, et al. Approaching a decade since HPV vaccine licensure: Racial and gender disparities in knowledge and awareness of HPV and HPV vaccine. Hum Vaccin Immunother 2017;13:2713-22. [Crossref] [PubMed]
- Cancer in North America (CiNA) Data Products [Internet]. North American Association Central Cancer Registries. 2018 [cited March 1, 2020]. Available online: https://www.naaccr.org/cina-data-products-overview/
- Definitions of risk factor-associated cancers. U.S. cancer statistics public use database [Internet]. Division of Cancer Prevention and Control, Centers for Disease Control and Prevention. 2020 [cited December 1, 2020]. Available online: https://www.cdc.gov/cancer/uscs/public-use/predefined-seer-stat-variables.htm
- Watson M, Saraiya M, Ahmed F, et al. Using population-based cancer registry data to assess the burden of human papillomavirus-associated cancers in the United States: overview of methods. Cancer 2008;113:2841-54. [Crossref] [PubMed]
- Damgacioglu H, Sonawane K, Zhu Y, et al. Oropharyngeal Cancer Incidence and Mortality Trends in All 50 States in the US, 2001-2017. JAMA Otolaryngol Head Neck Surg 2022;148:155-65. [Crossref] [PubMed]
- Yang DX, Soulos PR, Davis B, et al. Impact of Widespread Cervical Cancer Screening: Number of Cancers Prevented and Changes in Race-specific Incidence. Am J Clin Oncol 2018;41:289-94. [Crossref] [PubMed]
- Howlader N, Ries LA, Mariotto AB, et al. Improved estimates of cancer-specific survival rates from population-based data. J Natl Cancer Inst 2010;102:1584-98. [Crossref] [PubMed]
- SEER Cause-Specific Death Classification [Internet]. Division of Cancer Control and Populatiion Sciences, National Cancer Institute. 2020 [cited December 1, 2020]. Available online: https://seer.cancer.gov/causespecific/
- Economic Research Service. Rural Classifications. U.S. Department of Agriculture2019 December 12, 2020. Available online: https://www.ers.usda.gov/topics/rural-economy-population/rural-classifications/what-is-rural.aspx
- United States Census Bureau. 2010 Census regions and divisions of the United States: U.S. Department of Commerce; updated October 8, 2021. Available online: https://www2.census.gov/geo/pdfs/maps-data/maps/reference/us_regdiv.pdf
- Rogers SN, Vedpathak SV, Lowe D. Reasons for delayed presentation in oral and oropharyngeal cancer: the patients perspective. Br J Oral Maxillofac Surg 2011;49:349-53. [Crossref] [PubMed]
- Seoane J, Takkouche B, Varela-Centelles P, et al. Impact of delay in diagnosis on survival to head and neck carcinomas: a systematic review with meta-analysis. Clin Otolaryngol 2012;37:99-106. [Crossref] [PubMed]
- Pitchers M, Martin C. Delay in referral of oropharyngeal squamous cell carcinoma to secondary care correlates with a more advanced stage at presentation, and is associated with poorer survival. Br J Cancer 2006;94:955-8. [Crossref] [PubMed]
- Tota JE, Best AF, Zumsteg ZS, et al. Evolution of the Oropharynx Cancer Epidemic in the United States: Moderation of Increasing Incidence in Younger Individuals and Shift in the Burden to Older Individuals. J Clin Oncol 2019;37:1538-46. [Crossref] [PubMed]
- Gillison ML, D'Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst 2008;100:407-20. [Crossref] [PubMed]
- D'Souza G, Cullen K, Bowie J, et al. Differences in oral sexual behaviors by gender, age, and race explain observed differences in prevalence of oral human papillomavirus infection. PLoS One 2014;9:e86023. [Crossref] [PubMed]
- Megwalu UC. Impact of County-Level Socioeconomic Status on Oropharyngeal Cancer Survival in the United States. Otolaryngol Head Neck Surg 2017;156:665-70. [Crossref] [PubMed]
- Megwalu UC, Ma Y. Racial disparities in oropharyngeal cancer survival. Oral Oncol 2017;65:33-7. [Crossref] [PubMed]
- Weng Y, Korte JE. Racial disparities in being recommended to surgery for oral and oropharyngeal cancer in the United States. Community Dent Oral Epidemiol 2012;40:80-8. [Crossref] [PubMed]
- Berger MH, Yasaka TM, Haidar YM, et al. Insurance Status as a Predictor of Treatment in Human Papillomavirus Positive Oropharyngeal Cancer. Laryngoscope 2021;131:776-81. [Crossref] [PubMed]
- Osazuwa-Peters N, Adjei Boakye E, Chen BY, et al. Association Between Head and Neck Squamous Cell Carcinoma Survival, Smoking at Diagnosis, and Marital Status. JAMA Otolaryngol Head Neck Surg 2018;144:43-50. [PubMed]
- Ho JY, Elo IT. The contribution of smoking to black-white differences in U.S. mortality. Demography 2013;50:545-68. [Crossref] [PubMed]
- American Lung Association. Tobacco Use in Racial and Ethnic Populations: American Lung Association; 2020. Available online: https://www.lung.org/quit-smoking/smoking-facts/impact-of-tobacco-use/tobacco-use-racial-and-ethnic
- Ucciferri C, Tamburro M, Falasca K, et al. Prevalence of anal, oral, penile and urethral Human Papillomavirus in HIV infected and HIV uninfected men who have sex with men. J Med Virol 2018;90:358-66. [Crossref] [PubMed]
- Prejean J, Song R, Hernandez A, et al. Estimated HIV incidence in the United States, 2006-2009. PLoS One 2011;6:e17502. [Crossref] [PubMed]
- Centers for Disease Control and Prevention. HIV Surveillance Report, 2018 (Updated); vol.31 May 2020. Available online: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html
- Hall HI, Byers RH, Ling Q, et al. Racial/ethnic and age disparities in HIV prevalence and disease progression among men who have sex with men in the United States. Am J Public Health 2007;97:1060-6. [Crossref] [PubMed]
- Gillison ML. Oropharyngeal cancer: a potential consequence of concomitant HPV and HIV infection. Curr Opin Oncol 2009;21:439-44. [Crossref] [PubMed]
- Beachler DC, Abraham AG, Silverberg MJ, et al. Incidence and risk factors of HPV-related and HPV-unrelated Head and Neck Squamous Cell Carcinoma in HIV-infected individuals. Oral Oncol 2014;50:1169-76. [Crossref] [PubMed]
- Centers for Disease Control and Prevention. Diagnoses of HIV infection in the United States and dependent areas 2019. HIV Surveillance Report, 2019;32. Available online: https://www.cdc.gov/hiv/library/reports/hiv-surveillance/vol-32/index.html
- Amini A, Jasem J, Jones BL, et al. Predictors of overall survival in human papillomavirus-associated oropharyngeal cancer using the National Cancer Data Base. Oral Oncol 2016;56:1-7. [Crossref] [PubMed]
- Martinez Tyson D, Medina-Ramirez P, Flores AM, et al. Unpacking Hispanic Ethnicity-Cancer Mortality Differentials Among Hispanic Subgroups in the United States, 2004-2014. Front Public Health 2018;6:219. [Crossref] [PubMed]
- Thompson CA, Gomez SL, Hastings KG, et al. The Burden of Cancer in Asian Americans: A Report of National Mortality Trends by Asian Ethnicity. Cancer Epidemiol Biomarkers Prev 2016;25:1371-82. [Crossref] [PubMed]
- Gupta A, Sonis S, Uppaluri R, et al. Disparities in Oral Cancer Screening Among Dental Professionals: NHANES 2011-2016. Am J Prev Med 2019;57:447-57. [Crossref] [PubMed]
- Vázquez-Otero C, Vamos CA, Thompson EL, et al. Assessing dentists' human papillomavirus-related health literacy for oropharyngeal cancer prevention. J Am Dent Assoc 2018;149:9-17. [Crossref] [PubMed]
- Hurley LP, O'Leary ST, Markowitz LE, et al. US Primary Care Physicians' Viewpoints on HPV Vaccination for Adults 27 to 45 Years. J Am Board Fam Med 2021;34:162-70. [Crossref] [PubMed]
- Lin L, Benard VB, Greek A, et al. Racial and ethnic differences in human papillomavirus positivity and risk factors among low-income women in Federally Qualified Health Centers in the United States. Prev Med 2015;81:258-61. [Crossref] [PubMed]
- Chaturvedi AK, Graubard BI, Broutian T, et al. Effect of Prophylactic Human Papillomavirus (HPV) Vaccination on Oral HPV Infections Among Young Adults in the United States. J Clin Oncol 2018;36:262-7. [Crossref] [PubMed]
- Daley EM, Vamos CA, Thompson EL, et al. The feminization of HPV: How science, politics, economics and gender norms shaped U.S. HPV vaccine implementation. Papillomavirus Res 2017;3:142-8. [Crossref] [PubMed]
- Nogues JC, Fassas S, Mulcahy C, et al. Human Papillomavirus-Associated Head and Neck Cancer. J Am Board Fam Med 2021;34:832-7. [Crossref] [PubMed]
Cite this article as: Villalona S, Stroup AM, Villalona S, Ferrante JM. Racial/ethnic disparities in HPV-related oropharyngeal cancer outcomes among males in the United States: a national cohort study. Ann Cancer Epidemiol 2022;6:4.


