
The prevalence of malignant melanoma in adults with mastocytosis and adnexal skin tumours: a case-control study
Introduction
Mastocytosis is a haematological disease that is characterized by the accumulation of aberrant mast cells. In cutaneous mastocytosis only the skin is affected, whereas at least one extra-cutaneous organ is involved in systemic mastocytosis, most often the bone marrow (1). The majority of patients with systemic mastocytosis also have skin involvement which presents as brown-livid maculae (2). Systemic mastocytosis is strongly associated with a somatic mutation in the KIT gene, which leads to increased proliferation and survival of the mast cell clone (3). Mastocytosis can cause a wide variety of mast cell mediator-related symptoms such as itch, flushing, gastrointestinal problems, anaphylaxis, osteoporosis and neurocognitive symptoms, as well as symptoms due to organ infiltration of mast cells (4). The prognosis is largely dependent on the subtype of systemic mastocytosis, with decreased survival chances for patients with aggressive subtypes (5).
Melanoma is a neoplastic disease of melanocytes, yet another KIT-bearing cell type that is present in the skin. Although melanoma is also associated with activating KIT mutations, these typically arise in other exons of the KIT gene than in mastocytosis (6). A population-based Danish study showed a hazard ratio of 7.5 (95% confidence interval 4.4–13.0) for melanoma among mastocytosis patients compared with the general population (7). A Swedish study of a cohort of adult mastocytosis patients in an expertise centre described that 4 out of 81 adult patients (5%) had developed a melanoma at some point in their lives (8). A control population was lacking in the second study. Furthermore, a few case reports of single patients who both had melanoma and mastocytosis have been published (9,10). However, these are all reports of an association without evidence for causality. Since most patients with mastocytosis frequently visit medical professionals including dermatologists, enhanced surveillance might be the main reason for the increased prevalence of melanoma that was found in the aforementioned studies, also known as Berkson’s bias. Comparison of a cohort with mastocytosis to another condition that necessitates dermatological treatment is therefore pivotal to avoid this detection bias.
The aim of this study was to further explore the possible association between mastocytosis and melanoma. Hereto, we performed a case-control study comparing a large cohort of adults with mastocytosis with a matched cohort of patients with adnexal skin tumours using the national pathology database. The term adnexal skin tumours encompasses a large group of neoplasms that are differentiated towards regular cutaneous adnexal structures such as hair follicles or sebaceous, apocrine or eccrine glands (11,12). They are most often benign and are mostly found in adults. Histopathological diagnosis is essential to distinguish adnexal skin tumours from other cutaneous neoplasms, which renders them an adequate control group for the current study which was based on pathology reports. Furthermore, we analysed a large cohort of adult patients from a large tertiary centre in order to find potential risk factors for melanoma. We present the following article in accordance with the STROBE reporting checklist (available at https://ace.amegroups.com/article/view/10.21037/ace-21-10/rc).
Methods
Participants
A search was conducted in the PALGA (Pathologisch Anatomisch Landelijk Geautomatiseerd Archief) database, in which all histopathology reports in The Netherlands are collected. The search was conducted between 1998 and 2018 because mastocytosis is being registered in the PALGA database since 1998. Only adults were included. The generic search terms that were used were: mastocytosis, mastocytoma, mast cell sarcoma, urticaria pigmentosa, melanoma and lentigo maligna. A case-control cohort was formed by conducting a similar search in the PALGA database for a diverse group of adnexal skin tumours. The search terms for this group were: adnexal tumour, follicular hamartoma, acanthoma, trichoepithelioma, trichoadenoma, trichoblastoma, trichilemmoma, pilomatricoma. This control disease was chosen because adnexal tumours are not known to be associated with an altered risk of melanoma, and they require histopathological confirmation of the diagnosis, rendering the PALGA database a representative measure. For each mastocytosis case, one age- and sex-matched control was randomly selected from the whole dataset of adnexal tumours using the PALGA software. Subsequently, the histopathology reports of both datasets were screened to ensure that each entry truly represented cases of mastocytosis, adnexal tumour or melanoma. For instance, cases with the conclusion ‘no signs of mastocytosis’ were removed from the dataset.
Because the PALGA database provides limited clinical patient data, we also analysed the prevalence of melanoma in the adult patient population of our mastocytosis centre to allow for the identification of potential individual factors associated with an increased risk of melanoma. The Erasmus MC is a tertiary medical centre in The Netherlands and centre of expertise for mastocytosis. All patients aged ≥18 years who presented with cutaneous or systemic mastocytosis between January 2009 and October 2020 were included. For the diagnosis of mastocytosis, the WHO criteria were used (1).
Measures and outcomes
The main outcome was the prevalence of melanoma in both cohorts. Additional characteristics were extracted from the PALGA database. The age and sex of each patient was registered as well as the time between both diagnoses, and the characteristics of the melanoma such as the subtype, location, Breslow thickness, the presence of ulceration and microsatellites, and TNM stage. The diagnosis of melanoma was considered to be synchronous with the mastocytosis or adnexal tumour when they were diagnosed within three months of each other. The Breslow thickness was given in millimetres. UV exposed skin areas were defined as the face, neck, and lower arms.
For the second cohort of our own centre, more detailed clinical data were available. Next to the characteristics described above, details on the subtype of mastocytosis, levels of serum tryptase and total IgE, D816V KIT mutational status, and the presence of other solid malignancies were extracted from the electronic patient files. These clinical variables were compared between the subgroups with and without melanoma to identify potential risk factors.
Lastly, the prevalence of melanoma in the PALGA groups (mastocytosis and adnexal skin tumors) as well as our own mastocytosis cohort were compared with the prevalence of melanoma in the general Dutch population, as extracted from the established CONCORD reports (13).
Ethical considerations
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was not necessary since all data were gathered retrospectively and anonymously, and participants were not subjected to any action for this study.
Statistical analysis
The PALGA software was used for the first search and to identify matched cases for age and sex in the control group of adnexal tumours. Comparison of the characteristics between both groups was performed using IBM SPSS Statistics version 25. A two-sided paired t-test was used to compare continuous variables and a chi square test for dichotomous variables. Prevalence rate ratios were calculated per 10,000 cases to compare the prevalence of melanoma in both cohorts and the general population using the available data. Lastly, for the Erasmus MC mastocytosis cohort, Mann Whitney U test was used to compare continuous variables between patients with or without melanoma, and Fisher’s exact for dichotomous variables.
Results
The risk of melanoma in mastocytosis
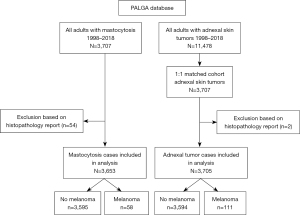
Between 1998 and 2018, a total of 5,509 reports on 3,707 individual patients in the PALGA database included the term ‘mastocytosis’. The large majority consisted of skin and bone marrow biopsies. After screening the entries for true cases of mastocytosis, 3,653 patients remained. From this selection, 58 patients had at least one report of melanoma (Figure 1). These encompassed 32 patients with mastocytosis in the skin with unknown status of systemic involvement, 25 patients with definite systemic mastocytosis and one patient with mast cell sarcoma. The prevalence of melanoma in this sample of mastocytosis patients was 1.6% and the prevalence rate ratio was 158.8 per 10,000 cases. A lifetime prevalence or hazard ratio cannot be calculated from these data, since the PALGA database does not provide information on the survival of patients.
Between 2000–2014, the mean prevalence rate ratio of all subtypes of melanoma in The Netherlands was 25 per 10,000 men and 30 per 10,000 women according to the CONCORD data (13). The prevalence rate ratio of melanoma is thus over 5× higher among adults with mastocytosis compared with the general population.
Melanoma in mastocytosis compared with adnexal skin tumours
The control group consisted of patients with different types of adnexal skin tumours extracted from the PALGA database between 1998–2018 and matched for age and sex with the mastocytosis patients. This produced a cohort of 3,707 unique patients (Figure 1). Two cases had to be excluded because of unreliable histopathology reports. Of the final population of 3,705 patients, 111 had at least one report of melanoma (3%, prevalence rate ratio 299.6 per 10,000 cases)
The characteristics of melanoma for both patient cohorts are summarized in Table 1. In 61.4% of mastocytosis patients, the melanoma was diagnosed prior to the diagnosis of mastocytosis, versus 41.9% in the group with adnexal tumours (P=0.026). Furthermore, a significantly larger proportion consisted of melanoma in situ in the mastocytosis group compared with the group of adnexal tumours (35% vs. 19.8%, respectively). There was a trend towards more synchronous diagnosis of melanoma in mastocytosis compared with adnexal tumours, although the absolute numbers were small in both patient populations. There was no statistically significant difference in the proportion of melanomas on UV exposed areas of the skin or Breslow thickness, but the number of patients with solid metastases was almost twice as high among patients with adnexal tumours than in the mastocytosis group without reaching statistical significance (17% vs. 8.8%, respectively, P=0.121).
Table 1
| Characteristic | Mastocytosis (n=58) | Adnexal tumor (n=111) | P |
|---|---|---|---|
| Age at first melanoma in years, mean (SD) | 52.3 (16.6) | 57.1 (16.7) | NS |
| Time between melanoma and mastocytosis/AT in years, mean (SD) | 7.5 (6.4) | 7.4 (5.0) | NS |
| Melanoma first, % | 61.4 | 41.9 | 0.026 |
| Synchronous diagnosis*, % | 7.0 | 4.5 | NS |
| UV exposed location, % | 22.8 | 30.7 | NS |
| Breslow thickness in mm, mean (SD) | 1.14 (1.0) | 1.22 (1.5) | NS |
| Type of melanoma, % | |||
| Superficial spreading | 47.4 | 56.3 | NS |
| Nodular | 8.8 | 6.3 | NS |
| Unknown primary# | 0 | 3.6 | NS |
| Lentigo maligna | 12.3 | 16.1 | NS |
| Melanoma in situ† | 35.0 | 19.8 | 0.036 |
| Miscellaneous | 1.7 | 1.8 | |
| Lymphatic metastases, % | 5.3 | 4.5 | NS |
| Solid metastases, % | 8.8 | 17.0 | NS |
| Multiple primary melanomas, % | 8.8 | 6.3 | NS |
| Other skin diseases than melanoma and mastocytosis/AT | 4x dysplastic melanocytic naevi 1x angiosarcoma | 5x BCC 3x actinic keratosis |
NS |
*, synchronous diagnosis: ≤3 months between two diagnosing biopsies; #, only reports of metastases were available; †, miscellaneous: 1 choroidal melanoma in mastocytosis cohort, 2 acrolentiginous melanomas in adnexal tumor group. AT, adnexal tumor; BCC, basal cell carcinoma; NS, not statistically significant.
Erasmus MC mastocytosis cohort
At the time of analysis, this cohort consisted of 269 adult patients with all subtypes of mastocytosis with a mean follow-up time of 13.9 years (SD 11.6). Eight out of 269 (3.0%) had developed a malignant melanoma at some point in their lives. This translates to a prevalence rate ratio of 297.4 per 10,000 cases which is almost 10× higher than the general Dutch population (prevalence rate ratio 25–30 per 10,000). Table 2 summarizes their characteristics. Patients with melanoma were significantly more often male (87.5% vs. 59%, P=0.03). One patient had melanoma in situ, and none had evidence of metastasis. In 4 patients (50%), mastocytosis of the skin was diagnosed co-incidentally when they presented with a physician for their melanoma. There was no statistically significant difference in the age of first symptoms of mastocytosis, serum tryptase levels at diagnosis, KIT mutation status, subtype of mastocytosis, or skin involvement between patients with or without melanoma.
Table 2
| Characteristic | Melanoma (n=8) | No melanoma (n=261) | P |
|---|---|---|---|
| Age at start mastocytosis in years, mean (SD) | 43 (17.0) | 40 (17.0) | NS |
| Male sex, % | 87.5 | 59 | 0.03 |
| Serum tryptase level at diagnosis in µg/L, mean (SD) | 42 (46.6) | 46.7 (61.6) | NS |
| Total IgE in kU/L, mean (SD) | 69 (211.0) | 31 (7.0) | NS |
| D816V mutation present, % | 87.5 | 83.2 | NS |
| Subtype of mastocytosis† | NS | ||
| Indolent systemic mastocytosis, % | 73 | 75 | – |
| Smoldering systemic mastocytosis, % | 1.9 | 0 | – |
| Advanced systemic mastocytosis, % | 9.2 | 12.5 | – |
| Cutaneous mastocytosis, % | 1.9 | 0 | – |
| Mastocytosis in the skin, % | 14.0 | 12.5 | – |
| Skin involvement of mastocytosis, % | 70.9 | 75 | NS |
| Other solid malignancy, % | 7.8 | 0 | <0.001 |
| Age at first melanoma in years, mean (SD) | 46 (19.9) | NA | – |
| Melanoma diagnosed before mastocytosis, % | 50 | NA | – |
| Follow-up after melanoma in years, mean (SD) | 10.5 (15.2) | NA | – |
| Synchronous diagnosis*, % | 50 | NA | – |
| UV exposed location†, % | 25 | NA | – |
| Melanoma in situ, % | 12.5 | NA | – |
| Lymphatic metastases, % | 0 | NA | – |
| Solid metastases, % | 0 | NA | – |
| Multiple primary melanomas, % | 0 | NA | – |
*, diagnoses within 3 months of each other; †, classification according to WHO criteria; Advanced systemic mastocytosis = aggressive systemic mastocytosis and systemic mastocytosis with associated hematological neoplasm. NA, not applicable; NS, not significant.
Discussion
Here, we found that the prevalence of melanoma is 1.6% in a large cohort of adults with histopathologically confirmed mastocytosis, and 3.0% in a smaller but representative patient cohort of the Erasmus MC, a national reference centre for mastocytosis. The prevalence rate ratio is 5–10 times higher than the prevalence of melanoma in the general Dutch population as reported by the CONCORD consortium (13), and comparable with previous reports from Scandinavian countries (7,8). Since patients with mastocytosis often visit physicians, and dermatologists in particular, enhanced skin surveillance might contribute to the higher prevalence of melanomas in this group, also known as Berkson’s bias. An adequate control population is therefore pivotal to correct for this potential bias. Patients with adnexal skin tumours were deemed to be an adequate control group since there was no known association with melanoma until now.
Interestingly however, the prevalence of melanoma proved to be even higher among patients with adnexal skin tumours than in the mastocytosis cohort, complicating the interpretation of the findings presented here. The prevalence rate ratio of melanoma was 10× higher in the adnexal skin tumour group compared with pre-existent data from the general Dutch population (13). The mastocytosis group had a higher proportion of in situ melanomas than the patients with adnexal tumours (35% versus 19.8%, respectively), and an almost twice lower rate of solid metastases (8.8% versus 17%, respectively. This suggests that enhanced skin surveillance and early detection of melanoma is an important factor in mastocytosis. However, melanoma was relatively often diagnosed prior to mastocytosis in the PALGA cohort and the diagnoses were made synchronously in 50% of the patients in our own centre. Improved detection of mastocytosis thus also happens in patients who present with a melanocytic lesion. It is well known that there is a considerable delay in the diagnosis of mastocytosis in adults, especially in the group that presents with skin involvement (14), and coincidental diagnosis of mastocytosis of the skin is not uncommon in our experience.
This study was not designed to investigate pathophysiological mechanisms that could contribute to the association between melanoma and mastocytosis. However, several hypotheses can be postulated on this topic. Mast cells are capable of producing several mediators that stimulate the proliferation of melanocytes (8,15,16). The increased levels of cell-free SCF in lesional skin of mastocytosis patients can also enhance melanocytosis (17). On the contrary, wild-type mast cells can probably inhibit the progression and invasion of melanomas (18,19). Accordingly, serum tryptase levels are lower in non-mastocytosis patients with aggressive melanoma compared with melanoma in situ or no melanoma (20). In our study, the melanomas appeared to behave less aggressively in the patients with mastocytosis than in the group with adnexal tumors. Although it is more likely that the higher proportion of melanomas in situ is a consequence of better dermatological surveillance, interaction of neoplastic mast cells with neoplastic melanocytes cannot be substantiated based on the current results.
While the primary goal of this study was to investigate the association between melanoma and mastocytosis, an unexpected yet interesting association between adnexal skin tumours and melanoma was identified. To our knowledge, no reports on this association have been published yet, although some types of adnexal tumours have been observed in cancer-prone syndromes such as familial adenomatous polyposis and Cowden syndrome (21). The fact that the prevalence of melanoma is not increased among patients with chronic skin diseases such as psoriasis (22) or atopic dermatitis (23) suggests that surveillance is not the only factor explaining the increased prevalence of melanoma in both cohorts. However, comparison of these diseases was not feasible using the PALGA database since psoriasis and atopic dermatitis usually do not require histopathological confirmation, while adnexal tumours mostly do.
This study has some limitations. Firstly, survival data are not available in the PALGA database, precluding the calculation of lifetime prevalence of melanoma. However, using a large cohort of patients and a matched control group enabled us to provide reliable information on the risk of melanoma. Secondly, the PALGA database does not include clinical data on the exact subtype of mastocytosis, tryptase levels, and other parameters. For this reason, we also investigated the patients in our mastocytosis referral centre, although the relatively low number of melanomas in this cohort renders it potentially underpowered for statistical analysis. Lastly, the PALGA database relies on the presence of histopathological diagnosis. It is unclear how many patients with mastocytosis are missing in the PALGA database because no biopsy of an affected organ was performed. However, the current cohort of 3,653 patients is considered large enough to be sufficiently representative.
Conclusions
The prevalence of melanoma was 1.6% in a large cohort of adults with mastocytosis, and 3.0% in a matched cohort of adnexal tumours. The prevalence rate ratio is approximately 5 and 10 times higher than in the general Dutch population, respectively. These results suggest that enhanced skin surveillance might, at least partly, lead to detection bias explaining the higher prevalence of melanoma among mastocytosis patients. While the primary goal of this study was to investigate the association between melanoma and mastocytosis, an unexpected yet interesting association between adnexal tumours and melanoma was identified. While this association may also be the consequence of Berkson’s bias, further research is necessary to corroborate these results and to identify a potential biological explanation. Until more is known about underlying pathophysiological mechanisms, it is recommended to inform patients with mastocytosis and adnexal skin tumours on the risk of melanoma and advise them to perform regular self-screening.
Acknowledgments
This work was supported by E.C. van den Broek PhD by means of technical assistance with the PALGA database. The authors would also like to thank L. Chaker, MD, PhD, for her advice on the statistical analysis.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://ace.amegroups.com/article/view/10.21037/ace-21-10/rc
Data Sharing Statement: Available at https://ace.amegroups.com/article/view/10.21037/ace-21-10/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ace.amegroups.com/article/view/10.21037/ace-21-10/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Because anonymized data were used for this retrospective study, ethics review or individual informed consent were not necessary according to local legislation.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Valent P, Akin C, Metcalfe DD. Mastocytosis: 2016 updated who classification and novel emerging treatment concepts. Blood 2017;129:1420-7. [Crossref] [PubMed]
- Hartmann K, Escribano L, Grattan C, et al. Cutaneous manifestations in patients with mastocytosis: Consensus report of the european competence network on mastocytosis; the american academy of allergy, asthma & immunology; and the european academy of allergology and clinical immunology. J Allergy Clin Immunol 2016;137:35-45. [Crossref] [PubMed]
- Chatterjee A, Ghosh J, Kapur R. Mastocytosis: a mutated KIT receptor induced myeloproliferative disorder. Oncotarget 2015;6:18250-64. [Crossref] [PubMed]
- Pardanani A. Systemic mastocytosis in adults: 2021 Update on diagnosis, risk stratification and management. Am J Hematol 2021;96:508-25. [Crossref] [PubMed]
- Sperr WR, Kundi M, Alvarez-Twose I, et al. International prognostic scoring system for mastocytosis (IPSM): a retrospective cohort study. Lancet Haematol 2019;6:e638-e649. [Crossref] [PubMed]
- Slipicevic A, Herlyn M. KIT in melanoma: many shades of gray. J Invest Dermatol 2015;135:337-8. [Crossref] [PubMed]
- Broesby-Olsen S, Farkas DK, Vestergaard H, et al. Risk of solid cancer, cardiovascular disease, anaphylaxis, osteoporosis and fractures in patients with systemic mastocytosis: A nationwide population-based study. Am J Hematol 2016;91:1069-75. [Crossref] [PubMed]
- Hägglund H, Sander B, Gülen T, et al. Increased risk of malignant melanoma in patients with systemic mastocytosis? Acta Derm Venereol 2014;94:583-4. [Crossref] [PubMed]
- Capo A, Goteri G, Mozzicafreddo G, et al. Melanoma and mastocytosis: is really only a coincidence? Clin Exp Dermatol 2019;44:76-7. [Crossref] [PubMed]
- Todd P, Garioch J, Seywright M, et al. Malignant melanoma and systemic mastocytosis--a possible association? Clin Exp Dermatol 1991;16:455-7. [Crossref] [PubMed]
- Alsaad KO, Obaidat NA, Ghazarian D. Skin adnexal neoplasms--part 1: an approach to tumours of the pilosebaceous unit. J Clin Pathol 2007;60:129-44. [Crossref] [PubMed]
- Obaidat NA, Alsaad KO, Ghazarian D. Skin adnexal neoplasms--part 2: an approach to tumours of cutaneous sweat glands. J Clin Pathol 2007;60:145-59. [Crossref] [PubMed]
- Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018;391:1023-75. [Crossref] [PubMed]
- Hermans MAW, Rietveld MJA, van Laar JAM, et al. Systemic mastocytosis: A cohort study on clinical characteristics of 136 patients in a large tertiary centre. Eur J Intern Med 2016;30:25-30. [Crossref] [PubMed]
- Welker P, Grabbe J, Gibbs B, et al. Human mast cells produce and differentially express both soluble and membrane-bound stem cell factor. Scand J Immunol 1999;49:495-500. [Crossref] [PubMed]
- Riechers A, Bosserhoff AK. Melanoma inhibitory activity in melanoma diagnostics and therapy - a small protein is looming large. Exp Dermatol 2014;23:12-4. [Crossref] [PubMed]
- Longley BJ Jr, Morganroth GS, Tyrrell L, et al. Altered metabolism of mast-cell growth factor (c-kit ligand) in cutaneous mastocytosis. N Engl J Med 1993;328:1302-7. [Crossref] [PubMed]
- Dyduch G, Okoń K, Pescarini E. Mast cells in melanocytic skin lesions. An immunohistochemical and quantitative study. Pol J Pathol 2011;62:139-44. [PubMed]
- Siiskonen H, Poukka M, Bykachev A, et al. Low numbers of tryptase+ and chymase+ mast cells associated with reduced survival and advanced tumor stage in melanoma. Melanoma Res 2015;25:479-85. [Crossref] [PubMed]
- Paolino G, Moliterni E, Didona D, et al. Serum tryptase levels in melanoma patients: case-control study and review of the literature. G Ital Dermatol Venereol 2019;154:18-25. [Crossref] [PubMed]
- Kanitakis J. Adnexal tumours of the skin as markers of cancer-prone syndromes. J Eur Acad Dermatol Venereol 2010;24:379-87. [Crossref] [PubMed]
- Wang L, Bierbrier R, Drucker AM, et al. Noncutaneous and Cutaneous Cancer Risk in Patients With Atopic Dermatitis: A Systematic Review and Meta-analysis. JAMA Dermatol 2020;156:158-71. [Crossref] [PubMed]
- Reddy SP, Martires K, Wu JJ. The risk of melanoma and hematologic cancers in patients with psoriasis. J Am Acad Dermatol 2017;76:639-647.e2. [Crossref] [PubMed]
Cite this article as: Hermans MAW, van Daele PLA, Damman J, van Doorn MBA, Pasmans SGMA. The prevalence of malignant melanoma in adults with mastocytosis and adnexal skin tumours: a case-control study. Ann Cancer Epidemiol 2022;6:1.


