
Pancreatic cancer mortality in Italy (1981–2015): a population-based study on geographic distribution and temporal trends
Introduction
Pancreatic cancer is the seventh leading cause of cancer deaths in industrialized countries globally and the fourth in Europe. It is a highly lethal malignancy with a 5-year survival rate of only 2–9%, mainly due to its rather symptomless progression until it reaches an advanced stage. Indeed, it is the most detected tumour during autopsy investigations. There are two main types of pancreatic cancer: the exocrine adenocarcinoma, which is the most common and lethal (85% of all cases), and the endocrine tumour (less than 5% of all cases) (1,2).
At global level, it is more frequent in men (5.5 per 100,000) than in women (4.0 per 100,000) (world standard population), with a higher incidence in white than in black people. Its occurrence increases with age: it is quite rare before age 40, but it starts to increase after 55 years, reaching its peak at about 70 years of age. The highest incidence and mortality rates have been detected in high-income countries in Europe and North America (7.7 and 7.6 per 100,000, respectively), while the lowest rates were found in Africa (2.2 per 100,000) (1-4). Within Europe, the highest mortality rates in males are reported from Eastern Europe and around the Baltic Sea, while the lowest are observed in Ireland, the United Kingdom, the Mediterranean countries (Italy included) and the Benelux. The highest rates for females are reported from Hungary, Malta and the Nordic countries, while the lowest rates are again found in the Mediterranean countries, excluding Italy (5-9).
Some recent studies carried out in Spain, Egypt, Brazil, and China analysed the geographic distribution of pancreatic cancer at subnational level; an uneven distribution of mortality rates within the national borders at regional, provincial, county or area levels was consistently detected (10-13). Pancreatic cancer aetiology is rather complex and still partially unknown. The International Agency for Research on Cancer (IARC) includes cigarette smoking, alcohol intake and red meat consumption among the risk factors causally associated with pancreatic cancer (2,6,14-16). Smoking, including passive exposure, represents the main risk factor. High alcohol consumption, i.e., more than three spirit drinks per day (excluding wine or beer), is also associated with increasing risk, probably due to alcohol-induced chronic pancreatitis. The synergistic effect of alcohol and smoke increases the risk of about four folds. Dietary factors associated with pancreatic cancer include, besides red meat, processed meat, poultry and fried food consumptions, whereas a protective role is attributed to vegetables, fruits and nuts. Obesity and inactivity, which may be considered mutually linked and connected to dietary habits, are also associated with an increased risk. As far as occupational exposure is concerned, pesticides, nickel and cadmium showed an association with pancreatic cancer. Unchangeable risk factors related to increased risk include diabetes mellitus (9.7% of all cases), Helicobacter pylori infection, chronic pancreatitis (10-fold higher than in general population), previous gastrectomy, genetic predisposition (10% of pancreatic cancer cases), family history (9-fold increased risk in individuals with a family history of pancreatic cancer), non-0 blood group (1,4,15,17-20).
A case-control study carried out in northern Italy between 1991 and 2008 (18) evidenced that 14% of occurrences were associated with tobacco smoking, 13% with heavy alcohol drinking, 12% with low adherence to the Mediterranean diet, 10% with diabetes and 1% with a family history of pancreatic cancer.
In most countries, pancreatic cancer incidence and mortality for both sexes have shown increasing trends for the last decades, particularly in less developed countries (1-5,9,11,21-23).
From 1990 to 2013, increasing temporal mortality trends were reported for both sexes in Spain. However, when focusing on the youngest age groups, increased mortality rates were detected exclusively in the female group, whereas a decrease was observed for males (10). In France, diverging incidence and mortality trends were detected between 1982 and 2012: incidence increased in both sexes, whereas mortality soared only in women (24). In Italy pancreatic cancer mortality rates for women also rose between 2003 and 2014 whereas its incidence substantially increased for men only (20,25).
A comprehensive investigation recently carried out in the European Union, revealed a steady rise in mortality for pancreatic cancer in both sexes between 1970 and 2015 in most member states, differently from all the other cancer types investigated. However, a projection to 2020 forecasted a levelling of this trend—at least in men—for Europe globally, even though with remarkable differences on a country-by-country basis (9). Other studies, however, have estimated an increase in the number of pancreatic cancers of more than 30% by 2040, just considering the ageing of populations (4,8).
On the basis of such a widespread rise of incidence and mortality rates from pancreatic cancer, its uneven geographic distribution within several countries, the poor prognosis and the still rather unclear aetiology, we decided to focus on its spatial and temporal mortality patterns in Italy between 1981 and 2015, at both national and municipal levels.
Due to the low survival time, mortality for pancreatic cancer is also considered a suitable incidence indicator. This study represents the first comprehensive investigation including all of the Italian municipalities, aimed at evidencing high risk areas in terms of both mortality levels and/or increasing temporal trends, possibly associating some risk indicators.
We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/ace-20-23).
Methods
Data sources
The present study has been carried out by the Italian National Agency for New Technologies, Energy and Sustainable Economic Development (ENEA) mortality database (data source: National Institute of Statistics—ISTAT), which includes all cause specific mortality records for Italian municipalities from 1980 to 2015. No ethical approval was required since the study is based on anonymous mortality data, not associable to specific individuals. Such data are officially provided to ENEA, already in the anonymous form, by ISTAT, that is the responsible agency for collection and codification of death certificates in Italy.
Specific pancreatic cancer mortality rates for males and females were calculated for the 35 years period 1981–2015, with reference to the 9th (1980–2002) and 10th (2003–2015) International Classifications of Diseases (ICD), whose codes for pancreatic cancer are 157 and C25, respectively.
Smoking habits and deprivation index (DI) were also taken into account as explanatory variables, possibly associated with pancreatic cancer mortality levels in each municipality. Alcohol consumption estimates for Italy are not available at municipal level; however, some descriptive comparisons based on regional data sourced from the “EpiCentro” website, maintained by the Istituto Superiore di Sanità (www.epicentro.iss.it/passi/) (26) were carried out.
Smoking habit indicators were estimated on the basis of a Multiscope survey on daily life aspects, conducted by ISTAT in 2015 on a sample of randomly selected families living in macro-areas (27). Smoker percentage (% smoker) was calculated as the ratio between the sum of smokers and ex-smokers, and the total of the interviewed participants for each macro-area (including up to five municipalities) and then assigned to the relative municipality.
The DI, based on 5 deprivation indicators—namely, the percentage of population with a lower education level, unemployment rates, the share of rented houses, mono-parental families and residential density—was chosen as continuous variable; the higher the value, the more deprived the municipality (28).
Statistical analysis
Age-specific mortality rates (ASpMRates) for pancreatic tumour for the overall 1981–2015 period in Italy were calculated separately for males and females, distributed into quinquennial age groups, up to the age of 74 years, and to a single, cumulative age-group for ages of 75 and above.
Pancreatic cancer standardized mortality rates per 100,000 (SMRates) (standard population source: 2011 Italian census), both for male and female residents in Italy above 35 years of age, and the relative 95% confidence intervals (95% CIs), were computed for the same period, both at national level and for all of the 8,116 Italian municipalities observed. Relative risks (RRs) compared to the mean SMRate for Italy were also calculated for each municipality.
The Italian ASpMRate and SMRate annual trends, and the septennial SMRate trends for each Italian municipality with at least 35 deaths due to pancreatic cancer during the period under consideration (673 and 625 municipalities for males and females, respectively) were calculated for the resident populations above age 35. The minimum deaths threshold was selected with a view to limiting casual fluctuations in SMRates and retaining a sufficient number of cases for temporal trend analysis. All trends were evaluated using the Joinpoint software (Joinpoint, RRID:SCR_018129) version 4.7.0.0, after logarithmic transformation of the ASpMRates or SMRates values. The values of the average annual percentage change (AAPC) or the average septennial percentage change (ASPC) were computed as an estimate of the trend magnitude. Graphs were realized using KyPlot software (5.0 version) and the maps were elaborated by QGIS software (QGIS, RRID:SCR_018507) version 3.6.
In order to identify significant associations between SMRates, % smokers and DI, we tested the feasibility of a multiple linear regression analysis using the statistical package SPSS (SPSS, RRID:SCR_002865) version 23, considering SMRate as the dependent variable in the model, and % smokers and DI as the explanatory variables.
Results
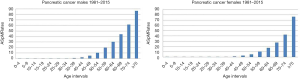
Pancreatic cancer ASpMRates in Italy for the period 1981–2015 are shown in Figure 1 by age-classes and gender. Pancreatic cancer appears as a typical disease of the elderly: it is extremely uncommon before age 30, whereas it starts to increase consistently after 40–45 years of age, reaching its peak in people older than 75 years.
Between 1981and 2015, deaths from pancreatic cancer in Italy were 137,972 for males and 137,432 for females, with SMRates of 16.15 (95% CI: 16.06–16.23) per 100,000 and 15.67 (95% CI: 15.58–15.75) per 100,000 respectively. In people older than 35 years, reported deaths amounted to 137,579 and 137,208 cases, with SMRates of 26.43 (95% CI: 26.29–26.57) per 100,000 and 23.78 (95% CI: 23.66–23.91) × per 100,000 for males and females, respectively.
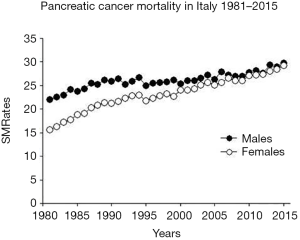
Figure 2 shows SMRate annual trends for males and females older than 35 years. A statistically significant increase (P<0.05) is evident in both sexes, even though it is steeper in female (AAPC: 1.41; 95% CI: 1.3–1.5) than in males (AAPC: 1.2; 95% CI: 1.0–1.4).
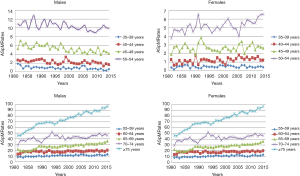
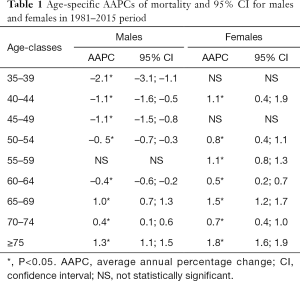
In Figure 3, annual trends of Italian ASpMRates in male and female populations older than 35 years are shown, and the relative parameters are reported in Table 1.
Full table
Indeed, negative trends are detected in males (excluding the 55–59 age class with P<0.05) and positive trends in females (excluding the 35–39 and 45–49 age classes with P<0.05).
Out of the 8,116 municipalities investigated in the 1981–2015 period, no pancreatic cancer death was observed in 495 municipalities for males and in 557 for females.
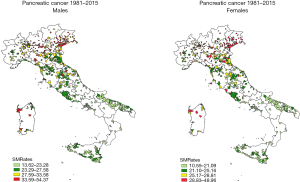
As far as the SMRate values are concerned, municipalities with more than 34 cases showed a non-homogeneous geographic distribution for both sexes (Figure 4). SMRates ranged from 13.62 (95% CI: 9.95–18.17) per 100,000 to 54.37 (95% CI: 38.55–74.78) per 100,000 in males, the highest occurring at Marmirolo (Mantua) in Lombardy region, in the north of Italy, and the lowest one in Ercolano (Naples) in Campania region, in the south of Italy. SMRates for females ranged from 10.55 (95% CI: 7.44–14.52) per 100,000 to 48.96 (95% CI: 35.37–66.03) per 100,000, the highest occurring in Castenedolo (Brescia), again in Lombardy region, and the lowest in Barcellona Pozza di Gotto (Messina) in the Sicily region, in the south of Italy. A decreasing gradient of SMRates from the north to the south of Italy is evident, with many municipalities with high mortality values in both sexes in Lombardy, Veneto, Friuli-Venezia Giulia, Trentino, Emilia-Romagna and Sardinia regions, followed by Piedmont, Liguria, Tuscany and Marche, and also Umbria only for males and Latium for females. In both sexes the lowest values refer to municipalities in Campania, Abruzzo, Molise, Apulia, Calabria, Basilicata and Sicily.
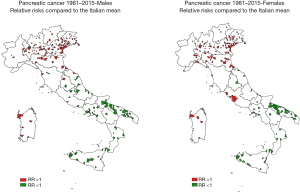
Considering the RRs referred to the Italian SMRates (Figure 5), a similar distribution pattern of municipalities among the Italian regions can be observed, but with an additional strong evidence of municipalities with higher female mortality in Piedmont, Liguria and Latium.
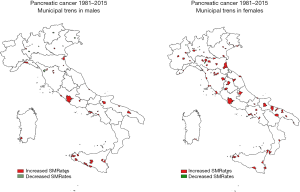
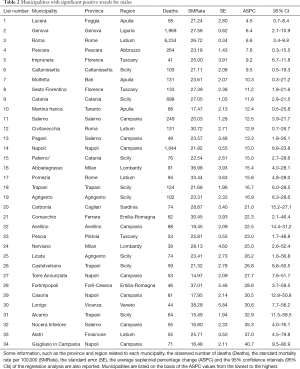
Temporal mortality trends for municipalities with at least 35 cases of death in the overall period (672 municipalities for males and 626 for females) were investigated. Statistically significant positive trends (P<0.05) were detected for 34 municipalities in males and for 84 municipalities in females whereas significant negative ones where observed for 11 and 5 municipalities, in males and females respectively (Figure 6). Most municipalities with increasing mortality trends are located in the south and central part of Italy for males, whereas they are more spread all over the peninsula for females. The negative trends are always located in the north of Italy. In Tables 2,3, a list of the municipalities with significant positive trends is reported. Some parameters such as the number of deaths, SMRates, ASPC and the 95% CI of the regression lines are also reported.
Full table
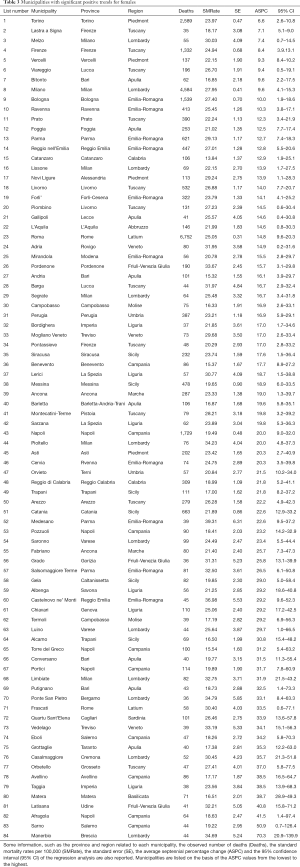
Full table
As far as the multiple linear regression analyses of SMRates, % smokers and DI values are concerned, the three indicators showed normal distributions. However, according to Pearson and Sign tests, very low correlation values were detected, indicating a poor correlation of the variables. Moreover, according to the Mahalanobis distance measure, multivariate normality conditions were not fulfilled and therefore the conditions of linearity among the dependent and independent variables were not satisfied.
Considering regional alcohol consumption habits, it is interesting to note an almost complete overlapping of the regions’ pattern with high alcohol consumption (in terms of several independent indicators related to alcohol consumption such as: yes or not, between meals, usually high, binge), with the regions we evidenced for the presence of municipalities with high or low SMRate or RR values (data not shown).
Discussion
The present study showed that Italy is aligned to most of the pancreatic cancer patterns reported from other European and non-European developed countries, such as the higher mortality in males and in the elderly, showing a consistent increase after the age of 40–45 and reaching a peak after the age of 74, and the general rise of mortality in both sexes (1-5,7).
The reasons for the higher mortality and incidence of pancreatic cancer in men than in women are still unknown but some hypotheses focus on different environmental or professional exposures by sex, lifestyle habits or genetic factors making women less susceptible than men (4).
The general increase of incidence and mortality observed in both sexes in the last few decades in developed countries whereas it still remains a relatively rare disease in less developed ones, could be partially explained by an improvement in the diagnostic procedures, the diffusion of unhealthy lifestyles (such as smoking and heavy alcohol consumption), coupled with the increasing age of populations and the consequent more frequent detection of a rather silent tumour at an early stage of development. However, recent data and future scenarios seem to indicate a sort of flattening of pancreatic cancer trends in some developed countries whereas a fast rising is forecasted in less developed countries (9,23,29). Although a specific reason for these differences can be hardly identified, it was hypothesised they could reflect changes in lifestyle habits such as smoking, which decreased in countries where health campaigning against tobacco smoking started earlier (1,2,4,9,21). However, pancreatic cancer is the only tumour, among many other tobacco-related malignancy, that has not shown consistent temporal declines in developed countries; moreover, the different pattern of tobacco smoking in subsequent generations cannot completely explain this phenomenon (9).
As far as temporal trends are concerned, we observed a steeper increase of mortality in females. This increment was also supported by the age-specific analysis which, besides a rise in mortality in both sexes for the higher age classes, showed an increase only among females in the lower age groups (up to age 64). Dramatically, this indicates that younger women account for the steeper mortality increase observed in females across Italy.
Similarly, evidence of a specific mortality increase in females has also been reported in France and in Spain and was then confirmed in Italy (10,24,25). In accordance with our results, the increase in female population mortality in Spain was particularly evident across the youngest age groups, whereas in males decreasing trends were observed (10).
During the observed period, no pancreatic cancer deaths were registered in 7% of the 8,116 Italian municipalities. Municipalities with at least 35 deaths from pancreatic cancer in the overall period, showed a general geographic gradient of mortality from northern to southern Italy, in terms of both SMRates and significant RRs, in agreement with incidence and mortality data reported by the Italian Association of Tumour Registries (20).
Sardinia strongly emerged as a region with high mortality levels for both sexes in some municipalities. This outcome should be carefully evaluated because the mortality levels and their geographic distribution are considerably different from that of the other southern and insular Italian regions.
Temporal trends at the municipal level showed statistically significant increases of mortality in 34 municipalities for males and 84 municipalities for females and significant decreases in 11 and 5 municipalities, respectively. Municipalities with negative trends were consistently found for both sexes in northern Italy; conversely, municipalities with positive trends were observed mainly in the southern and central regions of Italy for men, while they were spread all over the peninsula for females. On the basis of this outcome, it seems that a sort of levelling process is ongoing, with some municipalities exhibiting increasing trends in areas where mortality is still rather low, and some others showing decreasing trends where mortality is already high.
These observed patterns presumably reflect some changes in the distribution of certain risk factors: in some areas, for example, specific relevant exposures might have already reached their maximum levels. Moreover, a sort of balancing process between sexes seems also to be in place, due to the prevalence of municipalities with positive trends in females. This could be at least partially explained by the occurring changes in lifestyle habits between sexes, such as smoke and alcohol consumption.
In order to evaluate the impact of some important risk factors on the development of pancreatic cancer, we aimed to perform a multiple linear regression analysis using two indicators available for all municipalities (i.e., smoking habits and socioeconomic level of the population). However, the preliminary multivariate normality test did not allow to carry out such an analysis and the univariate correlation between pancreatic cancer SMRates and the two independent variables was very low. This outcome is probably due to the limited size of the population in several municipalities and, consequently, the wide CI of the SMRates they originate, resulting in a low accuracy of the estimates; moreover, the selected indicators might not be suitable enough to represent the underlying aetiological factors playing a role.
In order to override the lack of specific indicators for alcohol consumption at municipal level, we carried out a comparison of our data with those available at regional level, which showed an almost complete spatial correspondence of regions with high alcohol intake and the presence of municipalities with high SMRate or RR values. The same phenomenon was evident for regions with low alcohol consumption and the presence of municipalities with low mortality values. This observation, even though based on a rough comparison of data and maps, seems to support the importance of alcohol as an aetiological factor, and some further efforts in finding out a proper indicator at the municipal level should be undertaken.
Besides tobacco smoking, the importance of heavy alcohol intake as a risk factor for pancreatic cancer (attributable risk 13% and 14%, respectively), was also evidenced by the case-control study carried out in the provinces of Milan and Pordenone, in northern Italy (18).
In conclusion, the present study gives a picture of pancreatic cancer mortality in Italy from 1981 to 2015 in terms of geographic distribution, temporal trends, gender and age-specific differences from national to municipal levels. A steeper mortality increase was detected at national level for females and, differently from males, mainly attributable to younger women. A decreasing gradient of municipal mortality levels from the north to the south of Italy was detected, although a completely different picture appeared when considering increasing temporal trends, mostly located in the southern and central Italy, for males, and spread all over the peninsula, for females. The municipalities emerging from the present investigation in terms of high mortality and/or increasing mortality trends due to pancreatic cancer, as well as the territories where these municipalities are more concentrated, should be considered as priority areas for specific epidemiological investigations, and adequate resources should be devoted to further investigate the etiological factors involved.
As suggested by other authors (3), pancreatic cancer epidemiology may be key to better understand its aetiology and thus the starting point for developing future cancer control strategies.
Acknowledgments
Authors thank Dr. Francesca Pacchierotti (ENEA, Laboratory Health and Environment) for the critic revision of the manuscript and her invaluable advices; Mr. Carlo Bosco (ENEA, Central Administration) for his much needed informatic support; Dr. Nicola Caranci (Servizio Sovranazionale di Epidemiologia, ASL TO3, Grugliasco, TO and Agenzia Sanitaria e Sociale Regionale, Regione Emilia-Romagna) for supplying with the deprivation index indicators; Dr. Lidia Gargiulo (Direzione Centrale delle Statistiche Socio-demografiche e Ambientali, Italian National Institute of Statistics) for data on smoking habits extrapolated from a multi-scope investigation on daily life aspects; Mr. Mauro Pace and Ms. Maria Dweggah for their valuable editorial suggestions and proofreading.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/ace-20-23
Data Sharing Statement: Available at http://dx.doi.org/10.21037/ace-20-23
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ace-20-23). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. No ethical approval was required since the study is based on anonymous mortality data, not associable to specific individuals. Such data are officially provided to ENEA, already in the anonymous form, by ISTAT, that is the responsible agency for collection and codification of death certificates in Italy. Such data are then uploaded into the ENEA Epidemiological Mortality Database, which also contains the codified list of all Italian municipalities, the decennial census populations and the International Disease Classifications codes and a specific software aimed at calculating the suitable indices for epidemiological research studies.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol 2016;22:9694-705. [Crossref] [PubMed]
- . GBD 2017 Pancreatic Cancer Collaborators. The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 2019;4:934-47. [Crossref] [PubMed]
- Mahdavifar N, Mohammadian M, Ghoncheh M, et al. Pancreatic cancer in the world: an epidemiological review. WCRJ 2018;5:e1162
- Rawla P, Sunkara T, Gaduputi V. Epidemiology of pancreatic cancer: global trends, etiology and risk factors. World J Oncol 2019;10:10-27. [Crossref] [PubMed]
- Lucas AL, Malvezzi M, Carioli G, et al. Global trends in pancreatic cancer mortality from 1980 through 2013 and predictions for 2017. Clin Gastroenterol Hepatol 2016;14:1452-62.e4. [Crossref] [PubMed]
- Ferlay J, Colombet M, Soerjomataram I, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer 2018;103:356-87. [Crossref] [PubMed]
- Hasworth G, Hales J, Martinez F, et al. Pancreatic cancer trends in Europe: epidemiology and risk factors. Medical Studies 2019;35:164-71. [Crossref]
- Maisonneuve P. Epidemiology and burden of pancreatic cancer. Presse Med 2019;48:e113-23. [Crossref] [PubMed]
- Carioli G, Bertuccio P, Boffetta P, et al. European cancer mortality predictions for the year 2020 with a focus on prostate cancer. Ann Oncol 2020;31:650-8. [Crossref] [PubMed]
- Etxeberria J, Goicoa T, López-Abente G, et al. Spatial gender-age-period-cohort analysis of pancreatic cancer mortality in Spain (1990-2013). PLoS One 2017;12:e0169751 [Crossref] [PubMed]
- Barbosa IR, Santos CAD, Souza DLB. Pancreatic cancer in Brazil: mortality trends and projections until 2029. Arq Gastroenterol 2018;55:230-6. [Crossref] [PubMed]
- Jiang F, Chu J, Chen X, et al. Spatial distribution and clusters of pancreatic cancer mortality in Shandong Province, China. Sci Rep 2019;9:12917. [Crossref] [PubMed]
- Baum C, Soliman AS, Brown HE, et al. Regional variation of pancreatic cancer incidence in the Nile Delta region of Egypt over a twelve-year period. J Cancer Epidemiol 2020;2020:6031708 [Crossref] [PubMed]
- Raimondi S, Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an overview. Nat Rev Gastroenterol Hepatol 2009;6:699-708. [Crossref] [PubMed]
- McGuigan A, Kelly P, Turkington RC, et al. Pancreatic cancer: a review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol 2018;24:4846-61. [Crossref] [PubMed]
- International Agency for Research on Cancer (IARC). IARC Monographs on the Identification of Carcinogenic Hazard to Humans. List of Classification by cancer site. Available online: https://monographs.iarc.fr/wp-content/uploads/2019/07/Classifications_by_cancer_site.pdf (Accessed July 2019).
- Talamini R, Polese J, Gallus S, et al. Tobacco smoking, alcohol consumption and pancreatic cancer risk: a case-control study in Italy. Eur J Cancer 2010;46:370-6. [Crossref] [PubMed]
- Rosato V, Polesel J, Bosetti C, et al. Population attributable risk for pancreatic cancer in Northern Italy. Pancreas 2015;44:216-20. [Crossref] [PubMed]
- Capasso M, Franceschi M, Rodriguez-Castro KI, et al. Epidemiology and risk factors of pancreatic cancer. Acta Biomed 2018;89:141-6. [PubMed]
- Carmine Pinto e AIRTUM Working Group. Pancreas esocrino. In: Associazione Italiana di Oncologia Medica-Associazione Italiana Registri tumori (AIOM-AIRTUM). editors. I numeri del cancro in Italia 2018. Brescia: Intermedia Editore, 2018:157-61. Available online: https://www.registri-tumori.it/cms/sites/default/files/pubblicazioni/2018_NumeriCancro-operatori.pdf
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Ferlay J, Partensky C, Bray F. More deaths from pancreatic cancer than breast cancer in the EU by 2017. Acta Oncol 2016;55:1158-60. [Crossref] [PubMed]
- Saad AM, Turk T, Al-Husseini MJ, et al. Trends in pancreatic adenocarcinoma incidence and mortality in the United States in the last four decades; a SEER-based study. BMC Cancer 2018;18:688. [Crossref] [PubMed]
- Bouvier AM, Uhry Z, Jooste V, et al. Focus on unusual Rise in pancreatic cancer incidence in France. Int J Epidemiol 2017;46:1764-72. [Crossref] [PubMed]
- Crocetti E, Mancini S. Pancreatic cancer incidence rises also in Italy. Int J Epidemiol 2017;46:2090. [Crossref] [PubMed]
- Istituto Superiore di Sanita (ISS). Epicentro, Il portale dell'epidemiologia per la sanità pubblica. Italy: Passi (Progressi delle Aziende Sanitarie per la Salute in Italia). Available online: https://www.epicentro.iss.it/passi/ (Accessed January 2020).
- Italian National Institute of Statistics (ISTAT). Indagine multiscopo sulle famiglie. Aspetti della vita quotidiana. ISTAT, 2015.
- Caranci N, Biggeri A, Grisotto L, et al. The Italian deprivation index at census block level: definition, description and association with general mortality. Epidemiol Prev 2010;34:167-76. [PubMed]
- Wu W, He X, Yang L, et al. Rising trends in pancreatic cancer incidence and mortality in 2000-2014. Clin Epidemiol 2018;10:789-97. [Crossref] [PubMed]
Cite this article as: Uccelli R, Mastrantonio M, Altavista P, Sciortino M, Carletti R. Pancreatic cancer mortality in Italy (1981–2015): a population-based study on geographic distribution and temporal trends. Ann Cancer Epidemiol 2021;5:1.


