
The importance of health literacy on clinical cancer outcomes: a scoping review
Introduction
Health literacy (HL) refers to an individual’s ability to access, understand, appraise, and apply health related information (1). According to conservative estimates of the prevalence of low HL in Canada, only less than 12% of people aged 65 and older will not experience HL impairments throughout their lives and this challenge is widely felt across the globe (2,3). Health systems can be complicated to understand, leaving even highly educated individuals vulnerable when navigating their care (2). In an era of increasingly complex advances in oncology, even the small number of patients with high HL will struggle to understand and act on information about their diagnosis and treatments, leading to higher mortality and morbidity among this population (4). Given that cancer patients also experience immense emotional and psychological distress, further impairing their ability to retain and utilize health information, the challenge of low HL in the context of cancer care is significant and deserves immediate attention (5).
In past decades increased attention has been paid to HL as a factor influencing health behaviors and the use of preventive health care services (6). Low HL has been reported to be associated with several adverse clinical health outcomes including increased incidence of chronic illness, poorer intermediate disease markers, and less use of preventive health services (7). People with low HL are hospitalized more frequently, are less likely to undergo cancer screening, and more likely to have their cancer detected later (6,8). Although extensive research has been conducted to understand the effects of low HL on various health outcomes, there are no existing scoping reviews that comprehensively summarize the effects of HL in the context of cancer care delivery and clinical cancer-related outcomes. In addition, HL is an evolving concept with several definitions and measures (9). The purpose of this review is to identify and collate evidence on the known associations between HL and clinical cancer outcomes. In this review, clinical cancer outcomes are defined attitudes, knowledge and behaviors likely to affect engagement in cancer prevention, screening, and/or management activities and health care service-related outcomes (e.g., health services utilization, adherence/compliance, cost-related outcomes). A secondary purpose is to determine what HL measures are most commonly used in cancer HL research. We present the following article in accordance with the PRISMA extension for scoping reviews (PRISMA-ScR) reporting checklist (available at http://dx.doi.org/10.21037/ace-20-30).
Methods
A scoping review was conducted to summarize what is known about the association between HL and clinical cancer outcomes in the literature. Five electronic databases (Ovid Medline, Ovid Embase, EBSCO CINAHL PsychInfo, and ERIC) were searched by an information specialist. Ovid Medline, Ovid Embase and EBSCO CINAHL were searched as they broadly cover biomedical/health science literature. PsychInfo was searched to identify HL measurement tools, as it covers a wide range of survey instruments. ERIC was searched to discover additional HL literature, as it covers education related topics. Searches were limited to the English language and articles published between 1990 and March 1, 2020, as valid instruments to measure HL were not widely used until 1992. Database specific search strategies were developed using the following search terms: literacy [MeSH], health literacy [MeSH], computer literacy [MeSH], information literacy [MeSH, functional literacy, conceptual literacy, and numeracy for HL and cancer, neoplasms [MeSH], oncology [MeSH], tumor, and carcinoma for cancer. See Supplementary file 1 for detailed search strategies.
Citations were uploaded into EndNote for duplicate removal and exported into a spreadsheet. Citations were screened independently by a single reviewer in a two-stage process. Studies that did not meet inclusion criteria were excluded during title and abstract scan or full-text review as required. A small sample of articles (5%, N=180) were screened by two independent reviewers to assess inter-rater reliability and no discrepancies were identified. The literature suggests that a single reviewer process has no impact or a negligible impact on findings when performed by an experienced reviewer, which was the case for the present review (10).
Articles were included if they were English language research studies (observational or experimental), focused on associations between HL and cancer-related outcomes. Articles found in the gray literature were excluded because the focus of this review was on empirical evidence of association. Use of a validated instrument to measure HL was required. The study population included all patients, with or without a cancer diagnosis, across all cancer disease types. Data was extracted from full text articles and study characteristics were summarized using numeric and thematic analyses. Data was extracted using a standardized charting form developed by the reviewers, consisting of ten dimensions: jurisdiction, study purpose, study design, disease site, stage in cancer journey, sample, HL measures, outcomes, associations (results of the study), and authors’ conclusion. Inductive thematic coding was conducted to classify clinical cancer outcomes and a numerical summary of HL measures used was additionally performed.
Results
The search yielded 3,591 articles. After duplicate removal and title and abstract scan, the number of eligible articles was reduced to 249. Backward reference searches of 13 systematic reviews identified nine additional articles. Data was extracted from 146 articles (see Figure 1).
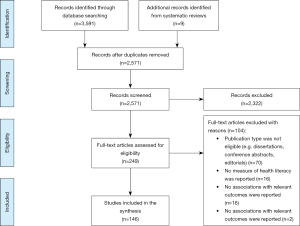
Numeric summary
Included studies were published in 20 countries, with most published in the US (N=91). Study design varied with a majority using cross sectional surveys (N=86). The top three cancer sites of interest were breast (N=51), cervix (N=25), and colorectal (N=32). The majority of studies focused on cancer in the screening stage (N=58), followed by prevention (N=27), treatment (N=26), survivorship (N=19), and diagnosis (N=4). Characteristics of included studies are summarized in Table 1.
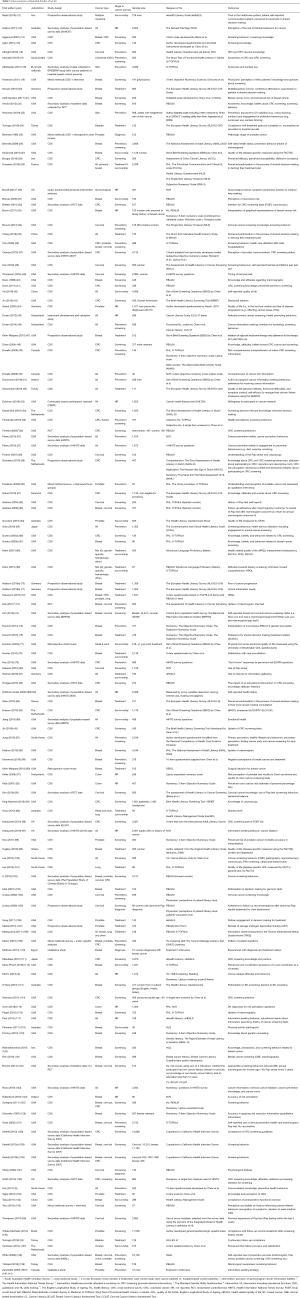
Full table
Thematic summary
HL assessment tools
Fifty-three HL measures were identified and thirty were validated. Measurement instruments are summarized in Table 2. Among these, nineteen measured general HL, nine numeracy, and eight functional HL.

Full table
The Rapid Estimate of Adult Literacy in Medicine (REALM) (N=24) and the Test of Functional HL in Adults (TOFHLA) including the abbreviated version (S-TOFHLA) (N=17) were most frequently used. Eighty-one studies examined general HL, 19 examined functional HL and 22 examined numeracy using. The three-item objective numeracy scale (126), which evaluates individuals’ understanding and ability to solve basic probability and related ratio problems (76), was the most frequently used (N=9).
Fifteen studies assessed cancer-specific literacy using eleven measurement tools. Echeverri et al. (53) used the Cancer Health Literacy Test as well as the Multidimensional Cancer Literacy Questionnaire. Another study used the Cancer Literacy Scale (45). Three studies assessed screening literacy using the Assessment of Health Literacy in Cancer Screening (38,73,93). The Cancer Message Literacy Test was used in two studies, to assess patients’ listening and reading abilities (25) and communication with physicians (142). Three studies assessed breast cancer related literacy (121,122,143). The Cancer Literacy Assessment Tool for breast cancer (B-CLAT) was used in the two studies (122,143) and it was adapted to assess cervical cancer literacy (143). One study used the Assessment of Colon Cancer Literacy measure (28).
Five studies assessed e-HL (12,81,111,117,144). The e-HL Scale was used in all five studies. Twenty-seven studies used other measures of HL: three used questionnaires combining a self-developed questionnaire and a validated HL instrument (44,145), and four used an indirect measurement of HL. Of the four, two used proxy measures (e.g., education, provider’s subjective assessment), in addition to objective measures (104,105). The other two studies used proxy variable data (e.g., education, television/internet use) extracted from the Health Information National Trends Survey (HINTS) in the US (39,83). Questions from population-based surveys were leveraged to collect sample specific data in ten studies. Among these, six used HINTS questions in their assessments of HL (39,57,79,86,123,132). One study used HL risks scores as an explanatory variable (122). Health risks scores were the sum of three indicators: low cancer literacy, no knowledge of family cancer history, and education less than 12 completed years (122). Five studies used author-developed questionnaires to measure HL (15,23,87,99,136). Three qualitative studies explored participants’ functional HL (110,140,141).
Clinical cancer-related outcomes
Outcomes were analyzed thematically and nine outcome categories were identified: preventive behaviors (N=55); cancer-related knowledge, awareness, attitudes, and beliefs (N=39); risk perception (N=9); information seeking (N=9); decision-making for cancer care (N=12); quality of life (QOL) (N=11); health status (N=6); post-treatment health behaviors (N=5); and provider-patient communication (N=6). Other outcomes included willingness to participate in cancer research (N=1) and trust in the healthcare system (N=1). Study outcomes are summarized in Table 3.

Full table
Preventive behaviors (N=55)
Cancer screening behaviors (N=42), were the most examined outcome. Eleven studies examined colorectal cancer (CRC) (17,22,28,56,79,82,97,114,128,146,147), (N=7) breast cancer (67,88,113,116,120,121,139) and (N=8) cervical cancer screening behaviors (34,38,39,62,80,93,105,142). Fourteen studies examined screening for 2 or more cancer types (14,36,46,55,63,73,74,102,122,125,127,129,130,138). Four studies examined general cancer screening (65,87,100,117). Most studies reported that individuals with adequate HL are more likely to participate in screening (N=30).
Six studies reported no significant association between HL and screening outcomes (14,63,66,113,117,125). One study found that adherence to breast and cervical cancer screening follow-up was similar regardless of the women’s functional HL status (63). Mixed results were reported in six studies (34,74,88,91,122,130). One study reported that HIV positive women with low HL were more likely to comply with cervical cancer screening recommendations, suggesting an inverse association with HL (P=0.02). However, the same women were less likely to meet annual screening recommendations in the long-term (P=0.05) (34). Alternate findings suggest that barriers to accessing health services, which are greater for those with inadequate HL (91), as well as the influence of ethnicity and language preference acculturation (88) may impact individuals’ uptake of screening services.
The association between HL and other preventative behaviours (e.g., fruit/vegetable consumption, exercise, smoking) was explored in eight studies (6,13,18,23,26,46,57,79). Six studies reported a positive association between higher levels of HL and engagement in preventative health behaviours. Adams et al. (13) reported that inadequate functional HL was associated with increased odds of reporting lifestyle risk factors (e.g., smoking, obesity, alcohol consumption). A structural equation model revealed that functional HL had a significant mediation effect on the path from socioeconomic status to perceptions of lifestyle risk factors (P<0.001) (13).
Two studies examining skin cancer prevention behaviours reported mixed findings, where HL was positively associated with health-promoting behaviours (e.g., sunscreen use), as well as non-health promoting behaviours (e.g., incidental UV exposure, sunless tanning) (P<0.05) (18,23). Fleary et al. (57) reported that HL was not a significant predictor for cancer prevention behaviors, although HL was positively associated with cancer prevention beliefs.
Two studies examined compliance with post-screening follow-up recommendations. The first found that that education and physician-estimated literacy level were significant predictors of duration of time to follow-up (P=0.005), whereas objective HL level was not (P=0.25) (105). The second reported that men with low HL were less likely to comply with follow-up recommendations after prostate specific antigen testing (P<0.0001) (136). A greater proportion of these men also had a higher probability of experiencing locally advanced prostate cancer (P<0.005) (136).
Three studies examined associations between HL and time to notice symptoms/seek medical help (25,110,135). One found that low-income men with low HL were more likely to present with more advanced stage prostate cancer when first seeking medical attention (P=0.02) (25). In a qualitative study exploring women’s experiences with diagnosis and treatment delays for breast cancer, even when women had adequate knowledge about breast cancer risk factors, they did not think they were at risk of cancer, which caused diagnostic delays (110). Tecu et al. (20) reported similar findings, however, they reported no statistically significant correlation association between HL and cervical cancer patient’s time to notice symptoms or time to decide to seek a medical help (135).
Two studies explored the relationship between HL and patients’ “Don’t Know” responses to risk perception questions. The first study reported that greater odds of “Don’t Know” responses were associated with lower knowledge of cancer prevention and screening strategies, lower health information seeking and lower numeracy (79), while the second conducted a path analysis and found no direct effects of HL (115).
Cancer-related knowledge, awareness, attitudes and beliefs
Thirty-nine studies reported relationships of HL with cancer-related knowledge, awareness, attitudes, and beliefs. Among these 26 studies reported positive associations with the outcomes and four reported negative associations (8,19,70,89). Three examined negatively worded variables as outcomes [e.g., negative perception of breast cancer treatment (89), fear of cancer progression (70), cancer fatalism (8)], so the inverse relations reported in these studies are considered positive. The last study reporting a significant negative association found that physicians’ numeracy level was negatively associated with their predictions of patients’ agreement to undergo regular mammography (P=0.012), implying that physicians with low numeracy tend to be more susceptible to biases and heuristics under uncertainty (19).
Four studies found no significant associations with the outcomes (21,34,66,82). One study reported no significant association between HL and cancer knowledge but found that women with lower HL were less likely to report having had a pap test within the past year (χ2=3.94, P=0.05) (34). Three studies reported mixed results (30,44,123). One found that the effectiveness of an educational intervention was moderated by HL, with smaller increases in symptom awareness, and smaller decreases in barriers to medical help seeking among female participants with lower HL (30). Another found that participants with low numeracy, expressed by discomfort with medical statistics, were more likely to report information overload, display fatalistic attitudes, lack cancer prevention knowledge, and worry about cancer more frequently (123). Other measures of perceived numeracy which measured understanding and use of health statistics were not associated with fatalism [odds ratio (OR) 1.22, 95% confidence interval (CI): 0.95–1.59, P=0.12], prevention knowledge (OR 1.11, 95% CI: 0.82–1.50, P=0.49), or high frequency of worry (OR 1.39, 95% CI: 0.95–1.80, P=0.59) (123). Two studies examined associations between HL and screening intention. One study reported a positive association (46), whereas the other study reported non-significant associations with fecal occult blood test intention (P=0.34) or colonoscopy intention (P=0.09) (32).
Friedman et al. (60) explored African American men’s understanding and misperceptions about prostate cancer risks and found that despite adequate HL levels, participant’s limited understanding and misperceptions were revealed during interviews and focus groups.
Risk perception
Nine studies examined perception of cancer risks (30,32,49,50,91-93,124,126). Most (N=7) explored how HL (numeracy) is associated with comprehension of cancer risk information. Although most reported positive correlations between HL and the outcomes, more complex findings were reported in the following two studies. Keller et al. (91) identified a significant (P<0.001) three-way interaction between format, risk level, and numeracy. Authors found that high-numerate participants could accurately interpret levels of risk presented in three different formats, while low-numerate participants could not observe differences between low-and high-risk scenarios in any format (91). Another study reported a negative association between HL and participant’s estimated risk of colon cancer, such that individuals with higher numeracy were likely to estimate lower personal percentage likelihood of developing colon cancer in their lifetime (92).
Information seeking
Nine studies examined information seeking outcomes that included the identification of unmet informational needs (71,108,117), information seeking (81,140,141), and odds of searching for cancer specific information (46,51,97). Four studies reported positive associations with HL (46,81,96,117). One reported that lower HL was associated with higher unmet needs (71). Another study found no significant association between HL and information needs, although education attainment was a significant predictor of information need (108).
Two qualitative studies examined patterns of information seeking strategies among patients with varying HL levels (140,141). Findings indicated that patients with high HL were more likely to consult a broad informational network for prostate cancer related information and support, while those with low HL were less likely to communicate their personal experience to individuals other than their urologist (140,141).
Decision making for cancer care
Twelve studies examined decision making for cancer care. Three examined decision making for screening (43,54,103), and nine explored treatment-related decision-making (12,20,35,47,76,84,90,107,144). Among these, six reported positive relationships between HL and the outcomes (12,20,35,76,84,144).
One study examined how the e-HL of partners of men with prostate cancer affects their involvement in treatment decision-making. The study found that partners’ e-HL was positively associated with active engagement in seeking a second opinion, awareness of treatment options, and use of a large social network for gathering information for treatment decision making (144). Lillie et al. (103) reported positive associations between breast cancer patients’ HL and their information-processing styles and preferences for active participation in the decisions regarding genomic tests. Women with higher HL indicated greater information retention, higher desire for medical information, and preferred more active participation in medical decision-making (103).
Three studies discussed how patients’ HL affects their decision for treatment options. The first found that the odds of undergoing early salvage androgen deprivation therapy, which is characteristic of undesirable effects, was greater among men with low HL and high prostate-specific antigen anxiety (107). Additionally, a study found that patients with low perceived HL were significantly less likely to undergo breast reconstruction following mastectomy (P=0.007), although no significant associations were found between HL and surgical treatment choice (P=0.89) (90). Keim-Malpass et al. (47) reported similar findings, such that a patient’s decision to begin adjuvant endocrine therapy for breast cancer treatment did not differ significantly across HL levels (P=0.426).
QOL
Eleven studies explored correlations between HL and QOL (27,42,52,64,68,69,72,77,85,99,100). Two studies found no significant differences in health related QOL between low and high literacy groups (68,69). Six studies reported positive effects of HL on the outcomes and two reported mixed results. In one study, HL acted as a moderating variable, such that breast cancer patients with lower levels of HL experienced significantly higher increases in their QOL when subject to cancer care coordination (27). Another study found that HL was positively associated with self-reported increases in general health, and negatively associated with self-reported symptoms and self-efficacy (P<0.001) (139).
Health status
Six studies examined self-reported health status including emotional and psychological health (44,83,86,95,99,131). Among these, three reported positive relations between HL and outcomes (44,83,131). One reported mixed results, such that poor HL measured by HeLMS was associated with high distress, while HL measured by the S-TOFHLA did not have a significant association with the distress level (P=0.74) (95). O’Hara et al. (113) examined the relationship between HL, emotional health, and mobile-based patient provider communication (MBPPC). HL was found to be positively associated with both MBPPC (β=0.09, P<0.05) and emotional health (β=0.12, P<0.05), while MBPPC was not significantly associated with emotional health. The Sobel test was conducted to support the mediation effect of HL (z=2.538, P<0.05) (86).
Post-treatment behaviours
Five studies examined post-treatment health behaviors, including self-reported level of physical activity among breast cancer survivors (118), post-operative functional exercise compliance among breast cancer patients (134), cystoscopy follow-up compliance among bladder cancer patients (24), and adherence to adjunctive endocrine therapy at 2-year follow-up (47). Four studies reported positive correlations between HL and the outcomes (24,118,134), while one reported non-significant results (47).
Provider-patient communication
Six studies explored associations between HL and outcomes in this category (12,78,112,137,142,146). One study reported that ovarian cancer survivors with low HL were more likely to report that they preferred receiving less information about medical tests and were less likely to be satisfied with information provided by their health care providers (137). Mixed results were found where low subjective numeracy was associated with perceiving low-quality provider communication, yet the association was reversed for objective numeracy (P<0.05) (146). The authors suggest that the mixed results can be explained by a discrepancy between confidence in numeracy skills and actual ability (146).
The third study found that patients with lower HL listening scores asked providers for personalized information while participants with higher scores asked a greater variety of risk/benefits questions. The authors suggest that this may imply that patients with lower HL listening skills have greater difficulty understanding information provided to them by their health care providers (142). Similarly, another study found that patients with higher HL scores were more likely to provide their oncologist with more information and asks more questions in comparison to lower HL counterparts (12).
Perception of care coordination was examined in two studies and one reported a positive association between HL and the outcome (78) and the other reported a negative association (P<0.001) (112). The latter result indicates that the use of a designated care coordinator may have a stronger influence on perceived care coordination in patients with lower HL (112).
Other outcomes
One study examined willingness to participate in cancer research and found that participants with higher HL were more likely to participate (53). Another study explored the relationship between e-HL and trust in the healthcare system (12). It was reported that individuals with higher HL have more trust in the healthcare system, which in turn increases their likelihood of participating in shared decision making (12).
One of two studies reported HL as an outcome found that membership in a prostate cancer support group was partially associated with HL, such that members of the support group were more likely to have greater knowledge about cancer, increased likelihood of having read guidelines relating to their diagnosis, and increased competency for health service navigation in comparison to non-support group member patients (145). The second found that the relationship between cancer-specific literacy and family communication varies, suggesting that family communication plays a role in health education and healthcare decision-making (143).
Six studies did not report direct associations between HL and relevant outcomes (147-152); however, findings suggest meaningful implications surrounding HL and cancer. For instance, Jenkins et al. (149) found that the experience of cancer diagnosis and treatment did not in itself impart greater health and cancer specific literacy, and Woudstra (152) identified eight decision-making stages and ten main HL skills for informed decision making in CRC screening. Additional findings are summarized in Table 4.
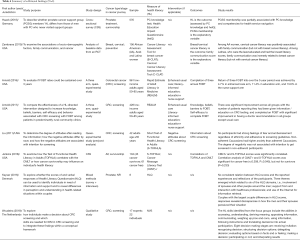
Full table
Discussion
The review identified associations between HL and clinical cancer-related outcomes. Among the outcome categories identified, Preventive Behaviors were most widely examined. The majority of studies reported positive associations between HL and clinical cancer related outcomes, suggesting that higher levels of HL are associated with a greater likelihood of experiencing favorable outcomes. The results show that inadequate HL is associated with: lower uptake of screening and preventative behaviours (22,43,116), longer lag time in symptom identification and medical help seeking (25,110), less knowledge of cancer and its prevention and treatment (40,48,120), impairments in risk perception (30,32,49), greater un-met informational needs (71), less information seeking behaviours (140,141), lower perceived QOL (27,85,99,100), less compliance with post-screening or post-treatment follow-up (24,134), and lower perceived quality and involvement in patient-provider communications (12,78,137). Implications of these associations are significant both for patients and the health care system at large. Individuals with low HL may present to the cancer system at more advanced stages of their disease or lack the skills required to self-manage their illness, leading to higher mortality and morbidity and greater care costs due to repeat emergency room visits and hospitalizations (11).
Mixed and contradictory findings were however also reported among the studies included in the review. Conflicting results may suggest implications surrounding measurement approaches for HL. Studies discussed that measurement instruments such as the REALM and TOFHLA may not be comprehensive measures of the broad range of skills and capabilities captured in the concept of HL (32,36). Experts agree that existing measures of HL are inadequate or incomplete, and that no existing measure holistically assess HL (55). This could explain the high between-study variability identified in the present review with respect to the tools and instruments used to assess HL.
Due to shortcomings of existing HL measures, the use of proxy measures or subjective assessment of HL was attempted in some studies, which may account for some of the conflicting outcomes reported. Several reports of mixed results were highlighted in studies that assessed both subjective and objective measures of HL (55,105,146). It is important to note, however, that it is unknown the extent to which proxy variables, subjective assessments, or self-reported HL may under or overestimate an individual’s actual HL level. For instance, literacy experts suggest that patients may attempt to hide their limited HL due to shame or social stigma (70). Individuals may also have a biased understanding of their own ability and skills related to health behaviors and health-related decision-making, which is then captured in their self-report data (37). Caution is therefore needed when interpreting HL results.
While this review was the first of its kind to summarize the effects of HL in the context of cancer care and clinical cancer-related outcomes, it is not without its limitations. A majority of studies included in the review employ a cross-sectional design, which does not allow for generalizability of findings or inferring causality between variables. Generalizability of findings is also limited across cancer stage and cancer type, as studies were largely focused on screening and prevention outcomes for patients with breast, cervical and CRC. The review was additionally limited to studies published in the English language and did not include articles published in the grey literature which may have led to the exclusion of relevant documents. Due to heterogeneity in the definition of HL in the literature, the search terms used in the present study to identify critical HL skills and capabilities may not have fully captured the construct. Another major limitation is that most data was based on participant self-report and therefore may be subject to social desirability and recall biases. Although this review presents supporting evidence for the association between HL and clinical cancer outcomes, more research is needed to explore these associations. As breadth of evidence is the focus of this review, methodological quality of the included studies was not assessed.
Conclusions
This review provides a detailed account of the associations between HL and clinical cancer in the literature. While a majority of studies reported a positive association between adequate levels of HL and favorable cancer-related outcomes, inconsistent results remain apparent. A high degree of variability in HL measurement tools is noteworthy and may account for some inconsistency in results. The findings of this review can be used to advance the field of cancer HL research by providing a clearer picture of the mechanics of HL and its impact on cancer health behaviours and preventative health care service use. Continued research in this area is critical as the promotion of HL among cancer patients has the potential to improve clinical outcomes and maintain good health.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA extension for scoping reviews (PRISMA-ScR) reporting checklist. Available at http://dx.doi.org/10.21037/ace-20-30
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ace-20-30). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolve.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sørensen K, Van den Broucke S, Fullam J, et al. Health literacy and public health: a systematic review and integration of definitions and models. BMC Public Health 2012;12:80. [Crossref] [PubMed]
- Kickbusch I, Pelikan JM, Apfel F, et al. Health literacy: the solid facts. Copenhagen, Denmark: World Health Organization Regional Office for Europe, 2013.
- Murray TS, Hagey J, Willms D, et al. Health Literacy in Canada: A Healthy Understanding. Canadian Council on Learning, 2008.
- Institute of Medicine (US) Committee on Health Literacy, Nielsen-Bohlman L, Panzer AM, Kindig DA. editors. Health Literacy: A Prescription to End Confusion. Washington (DC): National Academies Press (US), 2004.
- Koay K, Schofield P, Jefford M. Importance of health literacy in oncology. Asia Pac J Clin Oncol 2012;8:14-23. [Crossref] [PubMed]
- Son YJ, Kim SH, Kim GY, et al. Associations between Health Literacy, Cancer-Related Knowledge, and Preventive Health Behaviors in Community-Dwelling Korean Adults. J Health Commun 2017;22:999-1006. [Crossref] [PubMed]
- Berkman ND, Sheridan SL, Donahue KE, et al. Health literacy interventions and outcomes: an updated systematic review. Evid Rep Technol Assess (Full Rep) 2011;1-941. [PubMed]
- Morris NS, Field TS, Wagner JL, et al. The association between health literacy and cancer-related attitudes, behaviors, and knowledge. J Health Commun 2013;18:223-41. [Crossref] [PubMed]
- Hernandez LM. Measures of Health Literacy: Workshop Summary. Washington, DC: National Academies Press, 2009.
- Waffenschmidt S, Knelangen M, Sieben W, et al. Single screening versus conventional double screening for study selection in systematic reviews: a methodological systematic review. BMC Med Res Methodol 2019;19:132. [Crossref] [PubMed]
- Papadakos JK, Hasan SM, Barnsley J, et al. Health literacy and cancer self-management behaviors: A scoping review. Cancer 2018;124:4202-10. [Crossref] [PubMed]
- Nejati B, Lin CC, Aaronson NK, et al. Determinants of satisfactory patient communication and shared decision making in patients with multiple myeloma. Psychooncology 2019;28:1490-7. [Crossref] [PubMed]
- Adams RJ, Piantadosi C, Ettridge K, et al. Functional health literacy mediates the relationship between socio-economic status, perceptions and lifestyle behaviors related to cancer risk in an Australian population. Patient Educ Couns 2013;91:206-12. [Crossref] [PubMed]
- Aggarwal A, Speckman JL, Paasche-Orlow MK, et al. The role of numeracy on cancer screening among urban women. Am J Health Behav 2007;31:S57-68. [Crossref] [PubMed]
- Agho AO, Parker S, Rivers PA, et al. Health literacy and colorectal cancer knowledge and awareness among African-American males. Int J Health Promot Educ 2012;50:10-9. [Crossref]
- Albright AE, Allen RS. HPV Misconceptions Among College Students: The Role of Health Literacy. J Community Health 2018;43:1192-200. [Crossref] [PubMed]
- Almutairi KM, Alonazi WB, Alodhayani A, et al. A Cross-Sectional Assessment of Literacy and Awareness, Attitudes, and Beliefs About Colorectal Cancer and Its Screening in Riyadh Region. J Cancer Educ 2018;33:660-7. [Crossref] [PubMed]
- Altsitsiadis E, Undheim T, de Vries E, et al. Health literacy, sunscreen and sunbed use: an uneasy association. Br J Dermatol 2012;167:14-21. [Crossref] [PubMed]
- Anderson BL, Schulkin J. Physicians' perceptions of patients' knowledge and opinions regarding breast cancer: Associations with patient education and physician numeracy. Breast Care 2011;6:285-8. [Crossref] [PubMed]
- Heuser C, Diekmann A, Kowalski C, et al. Health literacy and patient participation in multidisciplinary tumor conferences in breast cancer care: a multilevel modeling approach. BMC Cancer 2019;19:330. [Crossref] [PubMed]
- April-Sanders A, Oskar S, Shelton RC, et al. Predictors of Breast Cancer Worry in a Hispanic and Predominantly Immigrant Mammography Screening Population. Womens Health Issues 2017;27:237-44. [Crossref] [PubMed]
- Arnold CL, Rademaker A, Bailey SC, et al. Literacy barriers to colorectal cancer screening in community clinics. J Health Commun 2012;17:252-64. [Crossref] [PubMed]
- Heckman CJ, Auerbach MV, Darlow S, et al. Association of Skin Cancer Risk and Protective Behaviors with Health Literacy Among Young Adults in the USA. Int J Behav Med 2019;26:372-9. [Crossref] [PubMed]
- Turkoglu AR, Demirci H, Coban S, et al. Evaluation of the relationship between compliance with the follow-up and treatment protocol and health literacy in bladder tumor patients. Aging Male 2019;22:266-71. [Crossref] [PubMed]
- Bennett CL, Ferreira MR, Davis TC, et al. Relation between literacy, race, and stage of presentation among low-income patients with prostate cancer. J Clin Oncol 1998;16:3101-4. [Crossref] [PubMed]
- Bennett IM, Chen J, Soroui JS, et al. The contribution of health literacy to disparities in self-rated health status and preventive health behaviors in older adults. Ann Fam Med 2009;7:204-11. [Crossref] [PubMed]
- McDowell BD, Klemp J, Blaes A, et al. The association between cancer care coordination and quality of life is stronger for breast cancer patients with lower health literacy: A Greater Plains Collaborative study. Support Care Cancer 2020;28:887-95. [Crossref] [PubMed]
- Boogar IR, Talepasand S, Norouzi H, et al. The prediction of colorectal cancer screening based on the extended parallel process model: Moderating the role of health literacy and cancer-related empowerment. Int J Cancer Manag 2018;11:e62539
- Ousseine YM, Durand MA, Bouhnik AD, et al. Multiple health literacy dimensions are associated with physicians' efforts to achieve shared decision-making. Patient Educ Couns 2019;102:1949-56. [Crossref] [PubMed]
- Boxell EM, Smith SG, Morris M, et al. Increasing awareness of gynecological cancer symptoms and reducing barriers to medical help seeking: does health literacy play a role? J Health Commun 2012;17:265-79. [Crossref] [PubMed]
- Brewer NT, Tzeng JP, Lillie SE, et al. Health literacy and cancer risk perception: Implications for genomic risk communication. Med Decis Making 2009;29:157-66. [Crossref] [PubMed]
- Brittain K, Christy SM, Rawl SM. African American patients' intent to screen for colorectal cancer: Do cultural factors, health literacy, knowledge, age and gender matter? J Health Care Poor Underserved 2016;27:51-67. [Crossref] [PubMed]
- Brown SM, Culver JO, Osann KE, et al. Health literacy, numeracy, and interpretation of graphical breast cancer risk estimates. Patient Educ Couns 2011;83:92-8. [Crossref] [PubMed]
- Bynum SA, Wigfall LT, Brandt HM, et al. Assessing the influence of health literacy on HIV-positive women's cervical cancer prevention knowledge and behaviors. J Cancer Educ 2013;28:352-6. [Crossref] [PubMed]
- Chang HL, Li FS, Lin CF. Factors Influencing Implementation Of Shared Medical Decision Making In Patients With Cancer. Patient Prefer Adherence 2019;13:1995-2005. [Crossref] [PubMed]
- Cho YI, Lee SY, Arozullah AM, et al. Effects of health literacy on health status and health service utilization amongst the elderly. Soc Sci Med 2008;66:1809-16. [Crossref] [PubMed]
- Ciampa P, Osborn C, Peterson N, et al. Impact of low numeracy on colorectal cancer screening utilization: results from the 2007 health information national trends survey (HINTS). J Gen Intern Med 2010;25:S303.
- Han HR, Kim K, Cudjoe J, et al. Familiarity, Navigation, and Comprehension: Key Dimensions of Health Literacy in Pap Test Use among Korean American Women. J Health Commun 2019;24:585-91. [Crossref] [PubMed]
- Thompson EL, Wheldon CW, Vamos CA, et al. How Is Health Literacy Related to Pap Testing Among US Women? J Cancer Educ 2019;34:789-95. [Crossref] [PubMed]
- Davis TC, Arnold C, Berkel H, et al. Knowledge and attitude on screening mammography among low-literate, low-income women. Cancer 1996;78:1912-20. [Crossref] [PubMed]
- Davis TC, Morris J, Rademaker A, et al. Barriers and Facilitators to Colorectal Cancer Screening Among Rural Women in Community Clinics by Heath Literacy. J Womens Health Issues Care 2017;6:1000292 [Crossref] [PubMed]
- Xia J, Wu P, Deng Q, et al. Relationship between health literacy and quality of life among cancer survivors in China: a cross-sectional study. BMJ Open 2019;9:e028458 [Crossref] [PubMed]
- Jin SW, Lee Y, Dia DA. Analyzing paths from online health information seeking to colorectal cancer screening using health literacy skills frame and cognitive mediation model. Patient Educ Couns 2019;102:416-23. [Crossref] [PubMed]
- Haack M, Kramer S, Seidel G, et al. Quality of life and fear of disease progression are associated with aspects of health literacy in men with prostate cancer from Germany. Support Care Cancer 2020;28:2283-92. [Crossref] [PubMed]
- Diviani N, Schulz PJ. First insights on the validity of the concept of Cancer Literacy: A test in a sample of Ticino (Switzerland) residents. Patient Educ Couns 2012;87:152-9. [Crossref] [PubMed]
- Diviani N, Schulz PJ. Association between Cancer Literacy and Cancer-Related Behaviour: Evidence from Ticino, Switzerland. J Public Health Res 2014;3:295. [Crossref] [PubMed]
- Keim-Malpass J, Doede A, Showalter SL. Does patient health literacy impact adherence to adjuvant endocrine therapy in breast cancer patients? Patient Prefer Adherence 2018;13:47-51. [Crossref] [PubMed]
- Dolan NC, Ferreira MR, Davis TC, et al. Colorectal cancer screening knowledge, attitudes, and beliefs among veterans: does literacy make a difference? J Clin Oncol 2004;22:2617-22. [Crossref] [PubMed]
- Donelle L, Arocha JF, Hoffman-Goetz L. Health literacy and numeracy: key factors in cancer risk comprehension. Chronic Dis Can 2008;29:1-8. [Crossref] [PubMed]
- Donelle L, Hoffman-Goetz L, Gatobu S, et al. Comprehension of Internet-based numeric cancer information by older adults. Inform Health Soc Care 2009;34:209-24. [Crossref] [PubMed]
- Drummond FJ, Reidy M, von Wagner C, et al. Health Literacy Influences Men's Active and Passive Cancer Information Seeking. Health Lit Res Pract 2019;3:e147-60. [Crossref] [PubMed]
- Ozkaraman A, Uzgor F, Dugum O, et al. The Effect of Health Literacy on Self-Efficacy and Quality of Life among Turkish Cancer Patients. J Pak Med Assoc 2019;69:995-9. [PubMed]
- Echeverri M, Anderson D, Nápoles AM, et al. Cancer Health Literacy and Willingness to Participate in Cancer Research and Donate Bio-Specimens. Int J Environ Res Public Health 2018;15:2091. [Crossref] [PubMed]
- Essink-Bot ML, Dekker E, Timmermans DRM, et al. Knowledge and Informed Decision-Making about Population-Based Colorectal Cancer Screening Participation in Groups with Low and Adequate Health Literacy. Gastroenterol Res Pract 2016;2016:7292369 [Crossref] [PubMed]
- Fernandez DM, Larson JL, Zikmund-Fisher BJ. Associations between health literacy and preventive health behaviors among older adults: findings from the health and retirement study. BMC Public Health 2016;16:596. [Crossref] [PubMed]
- Ferreira MR, Dolan NC, Fitzgibbon ML, et al. Health care provider-directed intervention to increase colorectal cancer screening among veterans: results of a randomized controlled trial. J Clin Oncol 2005;23:1548-54. [Crossref] [PubMed]
- Fleary SA, Paasche-Orlow MK, Joseph P, et al. The Relationship Between Health Literacy, Cancer Prevention Beliefs, and Cancer Prevention Behaviors. J Cancer Educ 2019;34:958-65. [Crossref] [PubMed]
- Fortner KB, Zite NB, Wallace LS. In my own words: Misunderstanding of pap smears and colposcopy among Appalachian women. J Low Genit Tract Dis 2007;11:251-7. [Crossref] [PubMed]
- Woudstra AJ, Smets EM, Dekker E, et al. Development and pilot-testing of a colorectal cancer screening decision aid for individuals with varying health literacy levels. Patient Educ Couns 2019;102:1847-58. [Crossref] [PubMed]
- Friedman DB, Corwin SJ, Dominick GM, et al. African American men's understanding and perceptions about prostate cancer: Why multiple dimensions of health literacy are important in cancer communication. J Community Health 2009;34:449-60. [Crossref] [PubMed]
- Gabel P, Larsen MB, Edwards A, et al. Knowledge, attitudes, and worries among different health literacy groups before receiving first invitation to colorectal cancer screening: Cross-sectional study. Prev Med Rep 2019;14:100876 [Crossref] [PubMed]
- Garbers S, Chiasson MA. Inadequate functional health literacy in Spanish as a barrier to cervical cancer screening among immigrant Latinas in New York City. Prev Chronic Dis 2004;1:A07. [PubMed]
- Garbers S, Schmitt K, Rappa AM, et al. Functional health literacy in Spanish-speaking Latinas seeking breast cancer screening through the National Breast and Cervical Cancer Screening Program. Int J Womens Health 2010;1:21-9. [PubMed]
- Goodwin BC, March S, Zajdlewicz L, et al. Health literacy and the health status of men with prostate cancer. Psychooncology 2018;27:2374-81. [Crossref] [PubMed]
- Goto E, Ishikawa H, Okuhara T, et al. Relationship of health literacy with utilization of health-care services in a general Japanese population. Prev Med Rep 2019;14:100811 [Crossref] [PubMed]
- Guerra CE, Dominguez F, Shea JA. Literacy and knowledge, attitudes, and behavior about colorectal cancer screening. J Health Commun 2005;10:651-63. [Crossref] [PubMed]
- Guerra CE, Krumholz M, Shea JA. Literacy and knowledge, attitudes and behavior about mammography in Latinas. J Health Care Poor Underserved 2005;16:152-66. [Crossref] [PubMed]
- Hahn EA, Cella D, Dobrez DG, et al. The impact of literacy on health-related quality of life measurement and outcomes in cancer outpatients. Qual Life Res 2007;16:495-507. [Crossref] [PubMed]
- Hahn EA, Garcia SF, Du H, et al. Patient attitudes and preferences regarding literacy screening in ambulatory cancer care clinics. Patient Relat Outcome Meas 2010;1:19-27. [Crossref] [PubMed]
- Halbach SM, Enders A, Kowalski C, et al. Health literacy and fear of cancer progression in elderly women newly diagnosed with breast cancer-A longitudinal analysis. Patient Educ Couns 2016;99:855-62. [Crossref] [PubMed]
- Halbach SM, Ernstmann N, Kowalski C, et al. Unmet information needs and limited health literacy in newly diagnosed breast cancer patients over the course of cancer treatment. Patient Educ Couns 2016;99:1511-8. [Crossref] [PubMed]
- Halverson JL, Martinez-Donate AP, Palta M, et al. Health Literacy and Health-Related Quality of Life Among a Population-Based Sample of Cancer Patients. J Health Commun 2015;20:1320-9. [Crossref] [PubMed]
- Han HR, Song Y, Kim M, et al. Breast and Cervical Cancer Screening Literacy Among Korean American Women: A Community Health Worker-Led Intervention. Am J Public Health 2017;107:159-65. [Crossref] [PubMed]
- Kim K, Han HR. The Association Between Health Literacy and Breast and Cervical Cancer Screening Behaviors: Findings From the Behavioral Risk Factor Surveillance System. Nurs Res 2019;68:177-88. [Crossref] [PubMed]
- Hanoch Y, Miron-Shatz T, Rolison JJ, et al. Understanding of BRCA1/2 genetic tests results: The importance of objective and subjective numeracy. Psychooncology 2014;23:1142-8. [Crossref] [PubMed]
- Hanoch Y, Miron-Shatz T, Rolison JJ, et al. Shared decision making in patients at risk of cancer: the role of domain and numeracy. Health Expect 2015;18:2799-810. [Crossref] [PubMed]
- Nilsen ML, Moskovitz J, Lyu L, et al. Health literacy: Impact on quality of life in head and neck cancer survivors. Laryngoscope 2020;130:2354-9. [Crossref] [PubMed]
- Hawley ST, Janz NK, Lillie SE, et al. Perceptions of care coordination in a population-based sample of diverse breast cancer patients. Patient Educ Couns 2010;81:S34-S40. [Crossref] [PubMed]
- Hay JL, Orom H, Kiviniemi MT, et al. "I don't know" my cancer risk: exploring deficits in cancer knowledge and information-seeking skills to explain an often-overlooked participant response. Med Decis Making 2015;35:436-45. [Crossref] [PubMed]
- Heberer MA, Komenaka IK, Nodora JN, et al. Factors associated with cervical cancer screening in a safety net population. World J Clin Oncol 2016;7:406-13. [Crossref] [PubMed]
- Heiman H, Keinki C, Huebner J. EHealth literacy in patients with cancer and their usage of web-based information. J Cancer Res Clin Oncol 2018;144:1843-50. [Crossref] [PubMed]
- Hodges NL, Shoben AB, Paskett ED, et al. Impact of a literacy-sensitive intervention on CRC screening knowledge, attitudes, and intention to screen. J Community Support Oncol 2016;14:420-6. [Crossref] [PubMed]
- Hoffman-Goetz L, Meissner HI, Thomson MD. Literacy and cancer anxiety as predictors of health status: An exploratory study. J Cancer Educ 2009;24:218-24. [Crossref] [PubMed]
- Shen HN, Lin CC, Hoffmann T, et al. The relationship between health literacy and perceived shared decision making in patients with breast cancer. Patient Educ Couns 2019;102:360-6. [Crossref] [PubMed]
- Husson O, Mols F, Fransen MP, et al. Low subjective health literacy is associated with adverse health behaviors and worse health-related quality of life among colorectal cancer survivors: Results from the profiles registry. Psychooncology 2015;24:478-86. [Crossref] [PubMed]
- Jiang S, Hong YA. Mobile-based patient-provider communication in cancer survivors: The roles of health literacy and patient activation. Psychooncology 2018;27:886-91. [Crossref] [PubMed]
- Jung SM, Jo HS, Oh HW. Internal Motivation, Perceived Health Competency, and Health Literacy in Primary and Secondary Cancer Prevention. Asian Pac J Cancer Prev 2016;17:5127-32. [PubMed]
- Kadivar H, Kenzik KM, Dewalt DA, et al. The association of english functional health literacy and the receipt of mammography among hispanic women compared to non-hispanic u.s.-born white women. PLoS One 2016;11:e0164307 [Crossref] [PubMed]
- Kamimura A, Chernenko A, Nourian M, et al. The Role of Health Literacy in Reducing Negative Perceptions of Breast Health and Treatment Among Uninsured Primary Care Patients. J Community Health 2016;41:858-63. [Crossref] [PubMed]
- Keim-Malpass J, Doede A, Camacho F, et al. Impact of patient health literacy on surgical treatment of breast cancer. Breast J 2018;24:633-6. [Crossref] [PubMed]
- Keller C, Siegrist M. Effect of risk communication formats on risk perception depending on numeracy. Med Decis Making 2009;29:483-90. [Crossref] [PubMed]
- Kelly KM, Graves KD, Harper FWK, et al. Assessing perceptions of cancer risk: Does mode of assessment or numeracy matter? Cancer Detect Prev 2007;31:465-73. [Crossref] [PubMed]
- Kim K, Xue QL, Walton-Moss B, et al. Decisional balance and self-efficacy mediate the association among provider advice, health literacy and cervical cancer screening. Eur J Oncol Nurs 2018;32:55-62. [Crossref] [PubMed]
- King-Marshall EC, Mueller N, Dailey A, et al. "It is just another test they want to do": Patient and caregiver understanding of the colonoscopy procedure. Patient Educ Couns 2016;99:651-8. [Crossref] [PubMed]
- Koay K, Schofield P, Gough K, et al. Suboptimal health literacy in patients with lung cancer or head and neck cancer. Support Care Cancer 2013;21:2237-45. [Crossref] [PubMed]
- Kobayashi LC, Wardle J, von Wagner C. Limited health literacy is a barrier to colorectal cancer screening in England: evidence from the English Longitudinal Study of Ageing. Prev Med 2014;61:100-5. [Crossref] [PubMed]
- Kobayashi LC, Smith SG. Cancer Fatalism, Literacy, and Cancer Information Seeking in the American Public. Health Educ Behav 2016;43:461-70. [Crossref] [PubMed]
- Koo K, Brackett CD, Eisenberg EH, et al. Impact of numeracy on understanding of prostate cancer risk reduction in PSA screening. PLoS One 2017;12:e0190357 [Crossref] [PubMed]
- Kugbey N, Meyer-Weitz A, Oppong Asante K. Access to health information, health literacy and health-related quality of life among women living with breast cancer: Depression and anxiety as mediators. Patient Educ Couns 2019;102:1357-63. [Crossref] [PubMed]
- Lee HY, Rhee TG, Kim NK. Cancer literacy as a mediator for cancer screening behaviour in Korean adults. Health Soc Care Community 2016;24:e34-42. [Crossref] [PubMed]
- Lee SH, Lee KH, Chang SJ. Do health literacy and self-care behaviours affect quality of life in older persons with lung cancer receiving chemotherapy? Int J Nurs Pract 2018;24:e12691 [Crossref] [PubMed]
- Li CC, Matthews AK, Dong X. The Influence of Health Literacy and Acculturation on Cancer Screening Behaviors Among Older Chinese Americans. Gerontol Geriatr Med 2018;4:2333721418778193 [Crossref] [PubMed]
- Lillie SE, Brewer NT, O'Neill SC, et al. Retention and use of breast cancer recurrence risk information from genomic tests: the role of health literacy. Cancer Epidemiol Biomarkers Prev 2007;16:249-55. [Crossref] [PubMed]
- Lindau ST, Tomori C, Lyons T, et al. The association of health literacy with cervical cancer prevention knowledge and health behaviors in a multiethnic cohort of women. Am J Obstet Gynecol 2002;186:938-43. [Crossref] [PubMed]
- Lindau ST, Basu A, Leitsch SA. Health literacy as a predictor of follow-up after an abnormal pap smear: A prospective study. J Gen Intern Med 2006;21:829-34. [Crossref] [PubMed]
- Song L, Tatum K, Greene G, et al. eHealth Literacy and Partner Involvement in Treatment Decision Making for Men With Newly Diagnosed Localized Prostate Cancer. Oncol Nurs Forum 2017;44:225-33. [PubMed]
- Mahal BA, Chen MH, Bennett CL, et al. High PSA anxiety and low health literacy skills: Drivers of early use of salvage ADT amongmen with biochemically recurrent prostate cancer after radiotherapy? Ann Oncol 2015;26:1390-5. [Crossref] [PubMed]
- Matsuyama RK, Wilson-Genderson M, Kuhn L, et al. Education level, not health literacy, associated with information needs for patients with cancer. Patient Educ Couns 2011;85:e229-36. [Crossref] [PubMed]
- Mazor KM, Rubin DL, Roblin DW, et al. Health literacy-listening skill and patient questions following cancer prevention and screening discussions. Health Expect 2016;19:920-34. [Crossref] [PubMed]
- McEwan J, Underwood C, Corbex M. Injustice! that is the cause: A qualitative study of the social, economic, and structural determinants of late diagnosis and treatment of breast cancer in Egypt. Cancer Nurs 2014;37:468-75. [Crossref] [PubMed]
- Mitsutake S, Shibata A, Ishii K, et al. Association of eHealth literacy with colorectal cancer knowledge and screening practice among internet users in Japan. J Med Internet Res 2012;14:e153 [Crossref] [PubMed]
- Mora-Pinzon MC, Chrischilles EA, Greenlee RT, et al. Variation in coordination of care reported by breast cancer patients according to health literacy. Support Care Cancer 2019;27:857-65. [Crossref] [PubMed]
- O'Hara J, McPhee C, Dodson S, et al. Barriers to Breast Cancer Screening among Diverse Cultural Groups in Melbourne, Australia. Int J Environ Res Public Health 2018;15:1677. [Crossref] [PubMed]
- Ojinnaka CO, Bolin JN, McClellan DA, et al. The role of health literacy and communication habits on previous colorectal cancer screening among low-income and uninsured patients. Prev Med Rep 2015;2:158-63. [Crossref] [PubMed]
- Orom H, Schofield E, Kiviniemi MT, et al. Low Health Literacy and Health Information Avoidance but Not Satisficing Help Explain "Don't Know" Responses to Questions Assessing Perceived Risk. Med Decis Making 2018;38:1006-17. [Crossref] [PubMed]
- Pagán JA, Brown C, Asch D, et al. Health literacy and breast cancer screening among Mexican American women in South Texas. J Cancer Educ 2012;27:132-7. [Crossref] [PubMed]
- Park H, Moon M, Baeg JH. Association of eHealth literacy with cancer information seeking and prior experience with cancer screening. Comput Inform Nurs 2014;32:458-63. [Crossref] [PubMed]
- Plummer LC, Chalmers KA. Health literacy and physical activity in women diagnosed with breast cancer. Psychooncology 2017;26:1478-83. [Crossref] [PubMed]
- Portnoy DB, Roter D, Erby LH. The role of numeracy on client knowledge in BRCA genetic counseling. Patient Educ Couns 2010;81:131-6. [Crossref] [PubMed]
- Rakhshkhorshid M, Navaee M, Nouri N, et al. The Association of Health Literacy with Breast Cancer Knowledge, Perception and Screening Behavior. Eur J Breast Health 2018;14:144-7. [Crossref] [PubMed]
- Roh S, Burnette CE, Lee YS, et al. Breast cancer literacy and health beliefs related to breast cancer screening among American Indian women. Soc Work Health Care 2018;57:465-82. [Crossref] [PubMed]
- Roman L, Meghea C, Ford S, et al. Individual, provider, and system risk factors for breast and cervical cancer screening among underserved Black, Latina, and Arab women. J Womens Health (Larchmt) 2014;23:57-64. [Crossref] [PubMed]
- Ross K, Stoler J, Carcioppolo N. The relationship between low perceived numeracy and cancer knowledge, beliefs, and affect. PLoS One 2018;13:e0198992 [Crossref] [PubMed]
- Rutherford EJ, Kelly J, Lehane EA, et al. Health literacy and the perception of risk in a breast cancer family history clinic. Surgeon 2018;16:82-8. [Crossref] [PubMed]
- Schapira MM, Neuner J, Fletcher KE, et al. The relationship of health numeracy to cancer screening. J Cancer Educ 2011;26:103-10. [Crossref] [PubMed]
- Schwartz LM, Woloshin S, Black WC, et al. The role of numeracy in understanding the benefit of screening mammography. Ann Intern Med 1997;127:966-72. [Crossref] [PubMed]
- Scott TL, Gazmararian JA, Williams MV, et al. Health literacy and preventive health care use among Medicare enrollees in a managed care organization. Medical Care 2002;40:395-404. [Crossref] [PubMed]
- Sentell T, Braun KL, Davis J, et al. Colorectal cancer screening: low health literacy and limited English proficiency among Asians and Whites in California. J Health Commun 2013;18:242-55. [Crossref] [PubMed]
- Sentell T, Braun KL, Davis J, et al. Health literacy and meeting breast and cervical cancer screening guidelines among Asians and whites in California. Springerplus 2015;4:432. [Crossref] [PubMed]
- Sentell TL, Tsoh JY, Davis T, et al. Low health literacy and cancer screening among Chinese Americans in California: A cross-sectional analysis. BMJ Open 2015;5:e006104 [Crossref] [PubMed]
- Sharp LK, Zurawski JM, Roland PY, et al. Health literacy, cervical cancer risk factors, and distress in low-income African-American women seeking colposcopy. Ethn Dis 2002;12:541-6. [PubMed]
- Smith SG, Kobayashi LC, Wolf MS, et al. The associations between objective numeracy and colorectal cancer screening knowledge, attitudes and defensive processing in a deprived community sample. J Health Psychol 2016;21:1665-75. [Crossref] [PubMed]
- Tagai EK, Miller SM, Kutikov A, et al. Prostate Cancer Patients' Understanding of the Gleason Scoring System: Implications for Shared Decision-Making. J Cancer Educ 2019;34:441-5. [Crossref] [PubMed]
- Tang W, Li Z, Tang C, et al. Health literacy and functional exercise adherence in postoperative breast cancer patients. Patient Prefer Adherence 2017;11:781-6. [Crossref] [PubMed]
- Tecu N, Potter P. Relationship of health literacy with women's cervical cancer knowledge and health behaviors. Journal of Gynecologic Oncology Nursing 2012;22:16-39.
- Tobias-Machado M, Carvalhal GF, Freitas CH Jr, et al. Association between literacy, compliance with prostate cancer screening, and cancer aggressiveness: results from a Brazilian screening study. Int Braz J Urol 2013;39:328-34. [Crossref] [PubMed]
- Verkissen MN, Ezendam NPM, Fransen MP, et al. The role of health literacy in perceived information provision and satisfaction among women with ovarian tumors: A study from the population-based PROFILES registry. Patient Educ Couns 2014;95:421-8. [Crossref] [PubMed]
- White S, Chen J, Atchison R. Relationship of preventive health practices and health literacy: a national study. Am J Health Behav 2008;32:227-42. [Crossref] [PubMed]
- Yılmazel G. Health Literacy, Mammogram Awareness and Screening Among Tertiary Hospital Women Patients. J Cancer Educ 2018;33:89-94. [Crossref] [PubMed]
- Zanchetta MS. Understanding functional health literacy in experiences with prostate cancer: Older men as consumers of health information. Online Brazilian Journal of Nursing 2004;3:4-15. [Crossref]
- Zanchetta MS, Perreault M, Kaszap M, et al. Patterns in information strategies used by older men to understand and deal with prostate cancer: An application of the modelisation qualitative research design. Int J Nurs Stud 2007;44:961-72. [Crossref] [PubMed]
- Mazor KM, Williams AE, Roblin DW, et al. Health literacy and pap testing in insured women. J Cancer Educ 2014;29:698-701. [Crossref] [PubMed]
- Zambrana RE, Meghea C, Talley C, et al. Association between Family Communication and Health Literacy among Underserved Racial/Ethnic Women. J Health Care Poor Underserved 2015;26:391-405. [Crossref] [PubMed]
- Song L, Tatum K, Greene G, et al. eHealth Literacy and Partner Involvement in Treatment Decision Making for Men With Newly Diagnosed Localized Prostate Cancer. Oncol Nurs Forum 2017;44:225-33. [PubMed]
- Haack M, Kofahl C, Kramer S, et al. Participation in a prostate cancer support group and health literacy. Psychooncology 2018;27:2473-81. [Crossref] [PubMed]
- Ciampa PJ, Osborn CY, Peterson NB, et al. Patient numeracy, perceptions of provider communication, and colorectal cancer screening utilization. J Health Commun 2010;15:157-68. [Crossref] [PubMed]
- Arnold CL, Rademaker A, Wolf M, et al. Final Results of a 3-Year Literacy-Informed Intervention to Promote Annual Fecal Occult Blood Test Screening. J Community Health 2016;41:724-31. [Crossref] [PubMed]
- Arnold CL, Rademaker A, Liu D, et al. Changes in Colorectal Cancer Screening Knowledge, Behavior, Beliefs, Self-Efficacy, and Barriers among Community Health Clinic Patients after a Health Literacy Intervention. J Community Med Health Educ 2017;7:497. [PubMed]
- Jenkins WD, Zahnd W, Spenner A, et al. Comparison of Cancer-specific and General Health Literacy Assessments in an Educated Population: Correlations and Modifying Factors. J Cancer Educ 2016;31:268-71. [Crossref] [PubMed]
- Liu CJ, Fleck T, Goldfarb J, et al. Attitudes to colorectal cancer screening after reading the prevention information. J Cancer Educ 2011;26:701-7. [Crossref] [PubMed]
- Kayser L, Hansen-Nord NS, Osborne RH, et al. Responses and relationship dynamics of men and their spouses during active surveillance for prostate cancer: health literacy as an inquiry framework. BMC Public Health 2015;15:741. [Crossref] [PubMed]
- Woudstra AJ, Timmermans DRM, Uiters E, et al. Health literacy skills for informed decision making in colorectal cancer screening: Perceptions of screening invitees and experts. Health Expect 2018;21:636-46. [Crossref] [PubMed]
Cite this article as: Samoil D, Kim J, Fox C, Papadakos JK. The importance of health literacy on clinical cancer outcomes: a scoping review. Ann Cancer Epidemiol 2021;5:3.


