
Cancer burden and control in Australia: lessons learnt and challenges remaining
Introduction
Cancer was the leading cause of disease burden in Australia in 2011 (1), which continues to rise due to the growth and ageing of the population along with some concurrent lifestyle behaviours associated with increased cancer risk (2). According to GLOBOCAN 2012 (3), Australia has among the highest age-standardised incidence rates (ASIR) of cancer (323 per 100,000). In this review article, we present an overview of the current cancer burden in Australia with measures of incidence, mortality and survival for all cancers combined, and the disparities in cancer burden by socio-economic disadvantage, geographic remoteness, country of birth and the “Aboriginal and Torres Strait Islander people” status. (The “Aboriginal and Torres Strait Islander peoples” are respectfully referred to as Indigenous Australians or people in this article). The discussion focusses on six selected major cancers to demonstrate Australia’s successes and shortfalls in cancer control through primary prevention, screening and early detection, as well as treatment. Comparisons are made between the current situation and that of two to four decades ago, and disparities among different population sub-groups are addressed.
Overview of cancer burden in Australia
Data sources
Cancer incidence and mortality data were sourced from the Australian Institute of Health and Welfare (AIHW) which collates cancer registry data from each Australian state and territory and assembles it into the Australian Cancer Database (ACD). The ACD includes all new cases of primary invasive cancer diagnosed in Australia from 1982 to 2014, except non-melanoma skin cancer (NMSC) as notification of these is not required by law.
Mortality data are also managed by AIHW within the National Mortality Database (NMD), which holds records for deaths in Australia and comprises information about causes of death with other characteristics of the person such as sex, age at death and Indigenous status. The deaths data are sourced from the Registrars of Births, Deaths and Marriages in each state and territory, the National Coronial Information System and are compiled and coded by the Australian Bureau of Statistics.
Statistics presented in this article have been extracted mostly from the AIHW website, in particular from the most recent publication “Cancer in Australia 2017” (2). The rest of the data were extracted from state based sources and various published articles.
Cancer incidence
In 2018, 138,321 new cases (54% males and 46% females) of all cancers are estimated to be diagnosed in Australia, compared to 134,174 in 2017. These predicted figures indicate an upward trend of new cancer cases from the reported incidence of 124,465 in 2013. It is also estimated that in 2017, for both males and females, 1 in 2 would be diagnosed with cancer by the age of 85 (2).
In contrast, the ASIR per 100,000 fell from 504 in 2008 to 483 in 2013 and is predicted to decrease to 470 in 2017. This decrease demonstrates a downward trend in cancer incidence rates during the last decade, and it has largely been observed for males and mainly reflects changes in the incidence rate for prostate cancer (2). The trends in incidence for all cancers combined as shown by the number of new cases versus the ASIR are presented in Figure 1.
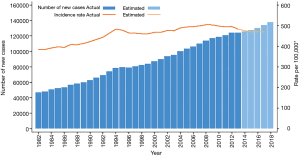
In 2018, it is predicted that the most commonly diagnosed cancer will be female breast cancer, followed by prostate cancer, colorectal cancer (CRC), melanoma of the skin and lung cancer. Also, in 2017, the 10 most commonly diagnosed cancers are estimated to account for 79% of all cancers diagnosed (2). These are listed in Table 1.
Table 1
| Position | (Estimated in 2018) | (Reported in 2011) | ||
|---|---|---|---|---|
| Incidence cases: cancer site (% total) | Mortality cases: cancer site (% total) | Burden of disease: cancer site (% total) | ||
| 1st | Female breast (13.2) | Lung (18.9) | Lung (18.6) | |
| 2nd | Prostate (12.8) | Colorectal (8.5) | Colorectal (11.1) | |
| 3rd | Colorectal (12.3) | Prostate (7.2) | Female breast (8.5) | |
| 4th | Melanoma of the skin (10.4) | Female breast (6.5) | Prostate (5.9) | |
| 5th | Lung (9.2) | Pancreas (6.2) | Pancreas (5.1) | |
| 6th | Lymphoma (4.6) | Unknown primary (5.8) | Brain & central nervous system (4.3) | |
| 7th | Leukaemia (2.8) | Liver (4.3) | Unknown primary (4.3) | |
| 8th | Kidney (2.6) | Melanoma of the skin (3.9) | Melanoma of the skin (4.2) | |
| 9th | Pancreas (2.4) | Leukaemia (3.9) | Leukaemia (3.7) | |
| 10th | Thyroid (2.4) | Lymphoma (3.0) | Other malignant neoplasms (3.6) | |
Source: AIHW ACD 2013.
By country of birth, the highest ASIR for all cancers for 2004–2008 in New South Wales, Australia’s most populous state, was for people born in New Zealand, followed by Australian-born, then those from Western Europe, Oceania and North America (4). Nationally, an average of 1,279 new cancer cases were diagnosed each year between 2009 and 2013 among Indigenous Australians which were 1.1 times more likely to be diagnosed than non-Indigenous Australians (5).
Cancer mortality
The estimated number of all cancer deaths in Australia is 48,586 for 2018, compared with 47,753 for 2017, which is equivalent to an average of 131 deaths each day. These predicted figures suggest an upward trend from the reported mortality of 44,171 in 2014 and are 1.9 times greater than for 1982. It was also estimated that in 2017 the risk of dying from cancer before the age of 85 will be 1 in 4 for males and 1 in 6 for females (2).
Conversely, the age-standardised mortality rate (ASMR) per 100,000 from all cancers combined was expected to decrease by 23% from 209 in 1982 to 161 in 2017. For males, the ASMR reached a peak in 1994 and decreased by 30% from 285 in 1994 to 200 in 2017. The trend can be largely attributed to declines in mortality rates for prostate cancer, CRC and lung cancer. Cancer mortality has been consistently lower for females than for males, and the ASMR remained fairly constant between 1982 and 1993, before decreasing by 21% from 164 in 1993 to 129 in 2017. This decrease can be largely attributed to the decline in the mortality rates for breast cancer and CRC (2). The mortality trends for all cancers combined shown by numbers of deaths and the ASMR are presented in Figure 2.

In 2018, lung cancer will be the leading cause of cancer mortality in Australia (9,198 deaths), followed by CRC [4,129], prostate cancer [3,500], female breast cancer [3,157] and pancreatic cancer [3,006]. These five cancers are expected to account for just under half (47.3%) of the total mortality from cancer in 2018, with lung cancer alone accounting for 1 in 5 (19%) cancer deaths (2). The 10 most common causes of cancer death are listed in Table 1.
Overall, the migrant groups (defined by country of birth) have more favourable cancer mortality rates compared to their Australian-born counterparts, with the notable exceptions of stomach and bladder cancers where some migrant groups have higher rates (6). There was an average of 551 cancer-related deaths each year between 2011 and 2015 for Indigenous Australians, who were 1.4 times higher than non-Indigenous Australians (5). Also, Indigenous Australians tend to have more advanced cancer at diagnosis across socio-economic disadvantage and remoteness groups (7), and remote-living Indigenous residents had higher risk of cancer death than Indigenous residents of metropolitan areas (8).
Cancer survival
According to GLOBOCAN 2012 (3), the mortality-to-incidence ratio (MIR) for Australia was 0.3 (while the world MIR was 0.6) for all cancers combined, suggesting that cancer survival in Australia was higher than for people in all other regions. Cancer survival in Australia remains among the highest in the world for most cancers (9). Between 2009 and 2013, in Australia, 5-year relative survival was highest for people diagnosed with testicular cancer (98%), thyroid cancer (96%) and prostate cancer (95%) and lowest for those diagnosed with pancreatic cancer (8%) and mesothelioma (6%). 5-year survival for all cancers combined increased from 48% in 1984–1988 to 68% in 2009–2013, and from 43% to 67.5% for males and from 55% to 68.7% for females during the same periods. The largest increases in survival were for prostate cancer, non-Hodgkin lymphoma, kidney cancer and multiple myeloma. There were only small survival improvements for pancreatic cancer and lung cancer; survival for cancers of the bladder and larynx decreased; and there was no change for lip cancer and mesothelioma (2). Disparities in cancer survival among different population sub-groups are discussed later.
Cancer burden
Cancer burden is measured as the combined impact of fatal and non-fatal burden. Fatal burden is expressed as years of life lost (YLL) due to premature death from cancer, while non-fatal burden is expressed as years lived with disability (YLD) due to cancer. The overall burden, which is expressed as disability-adjusted life years (DALYs), is the sum of YLL and YLD. In 2011, cancer was the leading cause of disease burden in Australia, with 833,250 DALYs lost (19% of total DALYs). Despite the high cancer survival rates in Australia in 2011, most of the cancer burden was fatal (94%), while only 6% of the burden was non-fatal (2).
Lung cancer was associated with the largest proportion of the cancer burden (19.0%), followed by CRC (11.0%), female breast cancer (8.5%), prostate cancer (5.9%) and pancreatic cancer (5.3%). Together, these five cancers accounted for almost half (49.4%) of the cancer burden in 2011. Despite improved survival for all these cancers since 1982, the burden from these five cancers was predominantly due to premature death (2). The top 10 major cancers with respect to total burden of disease are also presented in Table 1.
Lessons from the past and challenges remaining in the future
The increase in number of new cases and deaths for all cancers combined as reported (Figures 1,2) are largely due to the growth in size and ageing of the Australian population. Ongoing population growth and ageing are likely to further increase the number of new cancer cases and deaths thus increasing the demand for health services. Population screening programs (e.g., for breast and CRC) are likely to continue to contribute to the increasing number of new cases while improvements in early diagnosis with advanced diagnostic technologies, e.g. for breast cancer (10) and thyroid cancer (11) are likely to result in decreased mortality. Further, changes in population exposure to cancer risk factors will result in a change in cancer incidence and mortality. For example, increased prevalence of chronic infection with Hepatitis B and C viruses as a result of increased number of immigrants from Asia has led to an increase in incidence and mortality rates for liver cancer in Australia (12).
The decreasing ASIR can be attributed to successful prevention strategies such as tobacco control (e.g. lung cancer for men) and population screening programs (e.g., cervical screening program). Both prevention and treatment advances are important factors contributing to the downward trend in mortality, and there are several examples of new treatments for breast, CRC, testicular cancer, acute lymphoblastic leukaemia, and lymphomas (13,14).
Cancer control in Australia has been ranked among the most successful internationally (9). In addition to the recent advances in cancer diagnosis and treatment, some of which have been accessible in Australia, there are five important characteristics which are the pillars for supporting and sustaining the success of cancer control. Here we use the acronym “PERUN” to describe them:
- Prevention strategies—several prevention programs have been launched in Australia including tobacco control, sun protection and immunisation programs. Among them, tobacco control is one of Australia’s most noteworthy. Australia’s low smoking prevalence is the outcome of rigorous, persistent, and extensive public health efforts and actions from all levels of governmental and non-government organizations. Tobacco control has made an impact on cancer control in Australia, with subsequent reductions in the incidence rates for smoking-related cancers especially lung cancer (15,16). Other outstanding prevention strategies are the publicly funded National HPV (human papillomavirus) Vaccination Program (NHVP) introduced by the Australian government in 2007 (17), and the public campaigns promoting sun protection for skin cancer [launched in the early 1980s and have reduced the burden of melanoma across successive generations (18-20)]. These prevention programs are discussed in more details in the later sections.
- Early detection/screening programs—there are three national population-based screening programs in Australia, namely BreastScreen Australia, the National Cervical Screening Program (NCSP) and the National Bowel Cancer Screening Program (NBCSP), the first two were introduced in 1991 and the last in 2006. The aim of screening programs is to identify cancer at pre-cancerous or early stages in asymptomatic healthy individuals through early detection. Screening tests and recommended follow-up are available at no cost to the target group of average risk Australians for whom there is evidence that the screening provides the best balance of benefits to harms. These programs have each improved outcomes for the relevant cancers (21-23).
- Registries for cancer cases—notification of all cancers, except NMSC, is required by law in Australia to be reported to the cancer registry in each Australian state and territory. These registries enable population-based surveillance of cancer incidence and mortality, which facilitates policies and strategies for improving cancer prevention, screening and treatment.
- Universal healthcare—all Australian citizens and permanent residents are entitled to subsidized primary healthcare and prescription medications from the Australian Government Department of Human Services (known as Medicare Australia, a publicly funded, universal healthcare system that has been in place since 1975). This has reduced the financial barriers to accessing healthcare (though not entirely eliminating them) and allowed free access to high quality medical services and treatments as well as equity in cancer care.
- Non-government organizations’ (NGOs) contribution—several NGOs dedicated to cancer control (e.g., Cancer Council, National Breast Cancer Foundation, Prostate Cancer Foundation of Australia) have made substantial contributions by funding research projects, providing information, education and support services to those affected by cancer and their families, and being drivers for advocacy in public health.
Despite Australia’s efforts in cancer control, there are population sub-groups that experience poorer cancer outcomes. For example, disparities in cancer survival have been reported among disadvantaged population groups, as in many other countries (24-27). Specifically, disparities in cancer survival persisted for several cancer sites (CRC, stomach, liver, lung, breast and prostate) over 1996–2008 despite overall increases in cancer survival (27), and such disparities are widening over time by socio-economic status for several cancers (28). Also, because Australia is geographically vast, those living in remote and rural areas generally have poorer cancer survival than their metropolitan counterparts (29). An increasing gap has been observed between rural and urban areas for breast cancer survival, though no such increase was observed for other major cancers (30,31). Indigenous Australians diagnosed with cancer had a much lower five-year relative survival than that for non-Indigenous Australians in 2007–2014 (5). Conversely, people born outside Australia had a similar or lower risk of cancer death than Australian-born residents, possibly due to the “healthy-migrant effect” or loss of follow-up after returning to their home countries following a cancer diagnosis (28).
In the following section, six selected cancers are discussed to demonstrate some notable accomplishments in cancer control in Australia, as well as some shortfalls which still exist.
Lung cancer
Lung cancer was the fifth most commonly diagnosed cancer in Australia and similar to other developed nations, around 85% of lung cancer was attributable to tobacco smoking (32). The steady decline in lung cancer incidence and mortality over the past 40 years can mostly be attributed to reductions in smoking prevalence due to ongoing, and comprehensive tobacco control initiatives. Smoking prevalence peaked at an estimated 72% in the 1940s for Australian men and 30% in the 1970s for women (33). After the health hazards of smoking were documented in the U.S. Surgeon General’s report in 1967 (34), tobacco control initiatives in Australia have included mass media smoking cessation campaigns, restrictions on tobacco advertising, smoke-free public places, and increases in tobacco excise taxes, such that in 2016, smoking prevalence was at an historic low of 12.2% (35). Australia is a signatory to the World Health Organization Framework Convention on Tobacco Control and in 2016, was implementing 5 of the 7 MPOWER recommended tobacco reduction measures at the highest implementation level (36). Nevertheless, because of the 20-30-year lag between tobacco exposure and its effect on cancer incidence, lung cancer is still a major source of health burden and is the leading cause of cancer death in Australia (37).
Moreover, smoking rates differ across population sub-groups, with rates ranging from 20% to 30% among those with low socio-economic status, those living in remote and very remote areas, and Indigenous people (35), and as high as 66% among people with psychotic disorders (38). Consequently, lung cancer rates are higher for these sub-groups than for the general population (5,15,16,39). Resistance to population-wide tobacco control strategies means targeted interventions may be required to lower smoking rates in these groups, such as subsidized smoking cessation medications and behavioural interventions. Nevertheless, Australia continues to work towards more stringent population-based tobacco control measures. These include a planned series of annual tax increases to 2020 which will bring the tax component of tobacco products to the ≥75% of the retail price recommended by the WHO (36), and tobacco seller licensing schemes which will facilitate the enforcement of tobacco control laws (e.g., prohibition on sales to minors) (40).
Australia has seen small improvements in lung cancer survival rates [from 9% to 13% 5-year relative survival between 1984–1988 and 2009–2013 (2)], however, long-term survival after a lung cancer diagnosis remains very low [similar to the U.S. and elsewhere (41)]. Because the pathways to a lung cancer diagnosis are complex, it is often diagnosed at an advanced stage when curative treatment is not possible (42). Comorbidities and non-specific symptoms lead to diagnostic difficulty and delays (43,44). Optimizing the diagnosis and treatment pathways for lung cancer remains a challenge in Australia, however research aimed at facilitating early diagnosis and rapid referral is underway (45-47). Further, despite a universal health care system in Australia, disparities in lung cancer care have been identified for those living in rural and remote areas and for Indigenous people. For example, 5-year lung cancer-specific survival for Indigenous people was 11% compared with 16% for non-Indigenous people (2007–2014) (5). Both Indigenous people and/or people living remotely in Australia may have limited access to medical care, and to specialist thoracic surgical centres in particular (39,48,49). Barriers in access to the health system, whether geographic, cultural, or sociodemographic, contribute to delays in diagnosis and limit treatment options. Other factors, such as the impact of poorer general health and more comorbid conditions may also contribute to disparities in cancer survival among these groups (50), however one study reported that in one Australian state, Indigenous Australians were 35% less likely to receive active treatment for lung cancer compared to non-Indigenous Australians, even after accounting for comorbidities and stage of disease at diagnosis (51). The Australian government developed The National Aboriginal and Torres Strait Islander Cancer Framework in 2015 with the purpose of improving cancer survival outcomes for this group, and identified lung cancer as a priority (52).
Although primary prevention through tobacco control is likely to be the most effective long-term strategy for reducing the disease burden due to lung cancer, the full benefits of these interventions will not be realized for many years to come, and there is room for secondary prevention and advances in lung cancer treatments to improve survival rates over the coming decades. Lung cancer screening for high risk smokers is not yet recommended in Australia (53) but trials aimed at optimizing the benefits, harms, and cost-effectiveness of screening with low dose computer tomography are underway (54). Furthermore, a number of targeted therapies and immunotherapies (i.e., gefitinib, erlotinib, crizotinib, nivolumab) are now subsidized by the Australian government for use with certain types of lung cancer. These initiatives will hopefully lead to further gains in survival for lung cancer.
CRC
In Australia, CRC was the second most commonly diagnosed cancer in 2017 for both males and females, with estimated ASIR of 67.3 for males and 49.4 for females (2). The ASIR has decreased over time, as has the mortality rate, and this is generally attributed to the introduction in 2006 of population screening, through the NBCSP (55). However, an increase in the prevalence of risky behaviours as a result of changing lifestyle and dietary patterns could see an increased risk of CRC in the younger generations (56).
Nearly half (49.8%) of CRC cases in Australia are attributable to exposure to known modifiable risk factors (57) including tobacco use, alcohol use, overweight and obesity, insufficient physical activity, insufficient fibre intake, and red and processed meat intake (58). Other established risk factors, not included in Whiteman et al.’s analysis (57) but associated with an increased risk of CRC, are inflammatory bowel disease, adult attained height, antibiotic use, and exposure to ionising radiation. Factors that reduce the risk of CRC are taking calcium supplements, consumption of dairy products and wholegrains, and regular aspirin use (58). A small proportion of CRC cases are likely to be associated with hereditary syndromes (e.g., Lynch Syndrome and familial adenomatous polyposis) which increase the risk at younger age, but these account for only ~3% and well below 1% of the total number of new CRC cases, respectively (59,60).
Most CRC cases develop from colonic polyps over a period of many years, and early identification and removal of these pre-cancerous polyps, through screening techniques, can prevent cancer development (61). The NBCSP uses immunochemical faecal occult blood test (iFOBT) and is currently in a phased implementation process (55). By 2020, all eligible men and women aged 50–74 will have been invited to screen every 2 years. According to the latest AIHW report, 41% of eligible individuals participated in iFOBT screening in 2015–2016, with an 8% screening positivity rate (55). A recent analysis evaluated the impact of the NBCSP at observed and increased participation rates and found the NBCSP to be cost-effective at all participation levels, with a reduction in the number of incident cases, deaths and total annual CRC expenditure after 2030 (62). However, AIHW reports along with additional studies have highlighted the disparities in NBCSP participation based on gender, geographic location, Indigenous status, place of birth and language spoken at home (55,63-65). Efforts are now being made by government and NGOs to improve the participation by the general population and by targeted population sub-groups (66,67).
The 5-year relative survival for CRC has increased since the 1980s and was 70% in 2010–2014 for all Australians (55). However, socio-economic/geographic disparities in CRC survival have been reported in Australia over the past decade, either defined by area health services (68), rural vs metropolitan (29) or socio-economic groups (69), and more recent studies indicated that these socio-economic inequalities in cancer survival persisted over time (27). A study of surgical outcomes and survival for Indigenous and non-Indigenous people with CRC in New South Wales reported that Indigenous people had poorer survival rates although rates of surgical treatment, complications and follow-up colonoscopy were similar for the two groups (70).
Female breast cancer
Australia has one of the highest incidence rates worldwide for breast cancer and this was the most commonly diagnosed cancer for females in 2013 (71). In 2018 it is expected to surpass prostate cancer to become the most commonly diagnosed cancer (excluding NMSC), accounting for 18,235 new cases, compared with 5,371 in 1982. The ASIR for breast cancer had been increasing steadily from 1982 to 2014 (2). One of the explanations for such a rise is seemingly the improved detection from screening as shown by the temporal correlation between the rise in incidence and implementation of the screening program. The average age at first diagnosis of breast cancer is 61 years, and 79% of new cases are aged over 50 at diagnosis, which coincides with the inclusion of women aged over 50 in the screening program. With the introduction of BreastScreen Australia program in 1991, the ASIR started to increase more sharply until around 2000, and after which it increased at a slower rate (72). Other contributors to the rise could be increases in the prevalence of factors which increase the risk of breast cancer, such as late first pregnancy, nulliparity and lifestyle choices.
The falling ASMR especially over the last two decades, coupled with steady improvement in survival over the same period, is most likely due to advances in both primary and adjuvant therapy and the success of breast screening in earlier detection of cancer lesions (73,74). Other contributing factor could be due to the significant influx of immigrants over the last few decades since breast cancer mortality rates are reportedly lower for the majority of immigrant women than for Australian-born women (6). Although breast screening rates are generally lower for immigrant groups than for Australian-born women (64,65), mortality rates were falling gently or were relatively stable among them (6).
Among all the major cancers, breast cancer in Australia has a relatively low MIR, and its survival rates have been one of the highest in the world (75). However, the high survival rates have been reported to be not uniform across all population sub-groups. Within Australia, poorer survival has been reported for areas of greater socio-economic disadvantage and geographic remoteness (30,69,76,77). Greater understanding of the underlying causes of this is crucial to minimize disparities and improve breast cancer outcomes for all population groups.
As survival has improved, focus on outcomes in quality of life is becoming increasingly important. Recently, major advances in breast cancer management and new surgical techniques have been developed to reduce mastectomy rates, improve aesthetic outcomes and quality of life. Thus, more breast surgeons need to be trained in Australia to meet the challenge (78).
Prostate cancer
Prostate cancer has been the most commonly diagnosed cancer for men in Australia since 1989 (79) and was the most commonly diagnosed cancer for both sexes combined in 2013, and is also amongst the highest in the world (71). However, the incidence rate is expected to decline in 2018 and prostate cancer will become the second most commonly diagnosed cancer in Australia. This decline in incidence rate has contributed to the downward trend in ASIR for all cancers combined since 2008. From 1982 to 2014, the ASIR for prostate cancer went through a dramatic sequence of ‘ups and downs’ which is illustrated in Figure 3. This trend from 1989 onwards could be explained by the rapid adoption of prostate-specific antigen (PSA) screening by medical practitioners following the listing on Australia’s Medicare Benefits Schedule in 1988, which led to the incidence rates doubling by the early 1990s. Then, as PSA screening use stabilized and the pool of latent prostate cancer cases in the population was depleted, incidence fell at a similar rate to its previous rise (80). There was a subsequent increase in incidence which peaked around the year 2009, possibly driven by the adoption of extended biopsy procedures, followed by a slower decline since then (81).
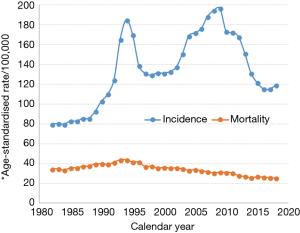
The ASMR was constant until the early 1990s (Figure 3), and then began a moderate and steady decline. This fall started around 4 years after the beginning of widespread PSA testing, and 5–6 years earlier than expected if screening was solely responsible. Also, a similar fall was observed in the United Kingdom, where PSA testing is less common. Thus, the reduction in the mortality rate cannot be explained solely by the increase in PSA testing (80).
Despite a notable drop (25%) in the ASMR between 1994 and 2011, Australian men experienced only half that observed (50%) for their American counterparts (82). It was also reported that cancer control measures in Australia generally result in better (for lung and CRC) or equal (for breast) outcomes than in the USA, with the exception of prostate cancer (3). Therefore, continued effort should be placed on developing evidence-based strategies to improve prostate cancer management in keeping with treatment, and ongoing follow-up.
5-year relative survival from prostate cancer was 94.5% during 2009–2013, showing significant improvements from 1984–1988 (2). However, there have been reports of ongoing disparities in prostate cancer survival by place of residence at diagnosis, with regional and rural Australian men having the poorest outcomes (31,83,84). In addition to improving overall prostate cancer survival in Australia to achieve comparability with that in USA and Canada, addressing inequity issues due to geographic variation is of utmost importance.
Cervical cancer
The major influence on cervical cancer incidence and mortality in Australia has been the introduction of the NCSP in 1991. Between 1991 and November 2017, the NCSP recommended two-yearly cytology-based screening for women aged 18–69 years; the recommendations were updated in December 2017 to five-yearly HPV-based screening for women aged 25–69 years. Cervical cancer incidence and mortality have fallen by approximately 50% and 45% respectively since the NCSP was introduced (85,86). While both incidence and mortality rates had been declining prior to 1991, probably due to opportunistic cervical screening, the declines following the introduction of the national organized program were far more pronounced (85,86); however these declines ceased in around 2004, and rates have been stable since then (85). In contrast, 5-year survival from cervical cancer has remained fairly stable over the period 1984–2013 (87). These patterns point to the key role which can be played by prevention in cervical cancer control. As a result of the clear aetiological role of HPV in the development of cervical cancer, major changes have occurred in cervical cancer prevention since 2006.
A NHVP was introduced in 2007, initially for females but also including males since 2013. Females born after ~1980 and males born after ~1998 have been offered the HPV vaccine. Uptake is over 70% for both males and females (88), and at this level indirect protection for unvaccinated individuals (“herd effects”) are expected to be substantial (89). Herd effects have been documented in Australia (90); for example the prevalence of vaccine-preventable HPV infections in unvaccinated women aged 18-35 years in 2015 was 87% lower than pre-vaccination levels (91). In 2018, the NHVP switched from a quadrivalent vaccine that protected against ~75% of cervical cancers in Australia, to a 9-valent vaccine that protects against ~90% (92,93).
Despite the overall success of the NCSP in Australia, there have been clear and persistent disparities in cervical cancer burden. Cervical cancer incidence and mortality are substantially higher for Indigenous women than for non-Indigenous women, and also vary by geographic area (87). Incidence and mortality rates increase with increasing levels of area-level disadvantage, and tend to be higher for women living in rural and remote areas than in major cities (87). These disparities in cervical cancer outcomes generally mirror similar disparities in two-yearly screening participation (87,94). Reassuringly, the impact of HPV vaccination appears to be similar across different levels of disadvantage (95), and to be at least as strong for the Indigenous Australians as for non-Indigenous Australians (96,97).
The NCSP was updated in December 2017 to recommend five-yearly HPV screening for women aged 25–69 years (with an exit test for women aged 70–74 years) (98), based on an extensive evidence review and a detailed modelled evaluation (99,100). The changes are predicted to decrease cervical cancer incidence and mortality by at least 20% (101). One important component of the updated NCSP is that women aged 30+ years who are never- or under-screened can be offered screening using a self-collected vaginal sample (98). Offering self-collection has been shown to increase screening participation among never- and under-screened women (102-104), and undergoing even one round of screening using self-collection would substantially reduce the cancer risk for unscreened women (105).
Together, the combination of HPV vaccination, self-collection, and screening that can be done less frequently are expected to help reducing the disparities that have so far persisted in cervical cancer in Australia, that appear to be related to disparities in participating in screening at the previously recommended frequency of every two years. Disparities could reduce even further in the future, as women in cohorts offered the 9-valent vaccine are likely to require even less frequent screening – potentially only once or twice in a lifetime (106-108).
Melanoma of the skin
Australia has one of the highest incidence rates of melanoma in the world (71), which is often referred to as “Australia's national cancer”. Melanoma is estimated to be the fourth most commonly diagnosed cancer in 2018, accounting for 14,320 new cases, 10.4% of all new cancers (2). In 2017, the risk of an Australian being diagnosed with melanoma by their 85th birthday was estimated to be 1 in 17 (1 in 13 males and 1 in 23 females). The ASIR had been increasing steadily for males and only slightly for females from 1982 to 2014. The ASMR had been relatively constant from 1982 to 2014 (2).
In Australia, the state of Queensland has the highest melanoma incidence rate in the world (109), due to the combination of a largely Caucasian population, high ambient UV radiation and an outdoor lifestyle (110). In the early 1980s, public campaigns promoting education and sun protection for skin cancer prevention and early detection began in Queensland, initially as the “Slip-Slop-Slap” campaign which was then replaced by the national SunSmart campaign in the early 1990s. Prevention and early detection campaigns for skin cancer have received wide coverage and continued in Queensland and nationally, with a strong focus on the childhood years (18,19).
There is strong evidence for the success of these campaigns in reducing the burden of melanoma even in this extremely high-risk population. Between 1995 and 2014 in Queensland, ASIR of melanoma continued to stabilize or decline for those aged under 40 years, has now stabilized for the 40–59 age group, and continues to increase only for people aged 60 years or over. Also, the ASMR decreased between 1995 and 2014 by 3.4% per annum for males under 40 and by 1.8% per annum for males aged 40–59. Mortality also fell by 2.9% per annum for females aged under 40 while it was stable for females aged 40 and over. The only group for whom there was a significant increase in mortality (by 1.8% per annum) was males aged 60 and over (20).
Nationally, the trends are consistent with those in Queensland. While melanoma is the most common cancer for adolescents and young adults (AYAs; age range of 15–24 years), the annual incidence rate for AYA decreased by 26% during 2000–2009 and by 35% during 2010–2011, and is projected to decrease by a further 17% in 2011–2020. The mortality rate has also decreased significantly by 71% between 1980–1989 and 2010–2012, with a further 8% reduction projected in 2013–2025. This is consistent with the increased 5-year survival rate from 86.3% in 1984–1988 to 90.4% in 2009–2013 (2).
Conversely, both the incidence and mortality rates were significantly higher for adults aged 60 and over in 2014, predominantly for males (2), which are most likely attributable to the accumulated UV radiation from sun exposure in their earlier life (prior to the 1980s). Further efforts are required to promote regular surveillance for early detection targeted at this age group, in addition to continuing and strengthening the public campaigns on sun protection.
Conclusions
Despite having implemented a number of cancer control initiatives, Australia continues to have a very high cancer burden. In this review, we focused on the six selected major cancers to highlight some cancer control successes and shortfalls in Australia. While some of the factors leading to Australia’s success in cancer control are quite distinctive in the world (e.g., universal healthcare), issues around the health inequities for Indigenous Australians and difficulties in access to health-care due to geographic remoteness remain despite longstanding efforts to close the gap. Health disparities among population sub-groups carry a substantial economic burden (111), thus it is worthwhile examining the causes of these disparities in cancer outcomes more thoroughly as they are not yet thoroughly understood. Ultimately, there is a need for commitments to strategy and action, matched with policy formulation, for real and sustainable change.
Acknowledgments
We gratefully acknowledge the support of Professor Dianne O’Connell for providing valuable comments on an earlier draft and Qingwei Luo for producing the graphs from the source.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Wanqing Chen) for the series “Global Cancer Burden” published in Annals of Cancer Epidemiology. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ace.2018.08.03). The series “Global Cancer Burden” was commissioned by the editorial office without any funding or sponsorship. XQY serves as an unpaid editorial board member of Annals of Cancer Epidemiology. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Australian Institute of Health and Welfare. Australian Burden of Disease Study: Impact and causes of illness and death in Australia 2011. Available online: https://wwwaihwgovau/getmedia/d4df9251-c4b6-452f-a877-8370b6124219/19663pdfaspx?inline=true, accessed 29/5/2018. 2016.
- Australian Institute of Health and Welfare. Cancer in Australia 2017. Cancer series no. 101. Cat. No.CAN100. AIHW: Canberra. Available online: https://wwwaihwgovau/getmedia/3da1f3c2-30f0-4475-8aed-1f19f8e16d48/20066-cancer-2017pdfaspx?inline=true, accessed 29/5/2018. 2017.
- Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1. 0, Cancer Incidence and Mortality Worldwide. Lyon, France: International Agency for Research on Cancer, 2014.
- Feletto E, Sitas F. Quantifying disparities in cancer incidence and mortality of Australian residents of New South Wales (NSW) by place of birth: an ecological study. BMC Public Health 2015;15:823. [Crossref] [PubMed]
- Australian Institute of Health and Welfare. Cancer in Aboriginal & Torres Strait Islander people of Australia. Available online: https://wwwaihwgovau/reports/cancer-screening/cancer-in-indigenous-australians/contents/incidence, accessed 29/5/2018. 2018.
- Anikeeva O, Bi P, Hiller JE, et al. Trends in cancer mortality rates among migrants in Australia: 1981–2007. Cancer Epidemiol 2012;36:e74-e82. [Crossref] [PubMed]
- Tervonen HE, Aranda S, Roder D, et al. Differences in impact of Aboriginal and Torres Strait Islander status on cancer stage and survival by level of socio-economic disadvantage and remoteness of residence-A population-based cohort study in Australia. Cancer Epidemiol 2016;41:132-8. [Crossref] [PubMed]
- Banham D, Roder D, Keefe D, et al. Disparities in cancer stage at diagnosis and survival of Aboriginal and non-Aboriginal South Australians. Cancer Epidemiol 2017;48:131-9. [Crossref] [PubMed]
- Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018;391:1023-75. [Crossref] [PubMed]
- Zhang L, Xu Y, Jin X, et al. A circulating miRNA signature as a diagnostic biomarker for non-invasive early detection of breast cancer. Breast Cancer Res Treat 2015;154:423-34. [Crossref] [PubMed]
- Vaccarella S, Dal Maso L, Laversanne M, et al. The impact of diagnostic changes on the rise in thyroid cancer incidence: a population-based study in selected high-resource countries. Thyroid 2015;25:1127-36. [Crossref] [PubMed]
- Amin J, O'Connell D, Bartlett M, et al. Liver cancer and hepatitis B and C in New South Wales, 1990–2002: a linkage study. Aust N Z J Public Health 2007;31:475-82. [Crossref] [PubMed]
- Yu XQ, O'Connell DL, Gibberd RW, et al. Trends in survival and excess risk of death after diagnosis of cancerin 1980–1996 in New South Wales, Australia. Int J Cancer 2006;119:894-900. [Crossref] [PubMed]
- Yu XQ, Chen WH, O'Connell DL. Improved survival for non-Hodgkin lymphoma patients in New South Wales, Australia. BMC Cancer 2010;10:231. [Crossref] [PubMed]
- Yu XQ, Luo Q, Kahn C, et al. Widening socioeconomic disparity in lung cancer incidence among men in New South Wales, Australia, 1987-2011. Chin J Cancer Res 2017;29:395-401. [Crossref] [PubMed]
- Yu XQ, Luo Q, Kahn C, et al. Contrasting temporal trends in lung cancer incidence by socioeconomic status among women in New South Wales, Australia, 1985-2009. Lung Cancer 2017;108:55-61. [Crossref] [PubMed]
- Garland SM. The Australian experience with the human papillomavirus vaccine. Clin Ther 2014;36:17-23. [Crossref] [PubMed]
- Stanton WR, Janda M, Baade PD, et al. Primary prevention of skin cancer: a review of sun protection in Australia and internationally. Health Promot Int 2004;19:369-78. [Crossref] [PubMed]
- Iannacone MR, Green AC. Towards skin cancer prevention and early detection: evolution of skin cancer awareness campaigns in Australia. Melanoma Manag 2014;1:75-84. [Crossref]
- Aitken JF, Youlden DR, Baade PD, et al. Generational shift in melanoma incidence and mortality in Queensland, Australia, 1995–2014. Int J Cancer 2018;142:1528-35. [Crossref] [PubMed]
- Roder D, Houssami N, Farshid G, et al. Population screening and intensity of screening are associated with reduced breast cancer mortality: evidence of efficacy of mammography screening in Australia. Breast Cancer Res Treat 2008;108:409-16. [Crossref] [PubMed]
- Ananda S, Wong H, Faragher I, et al. Survival impact of the Australian National Bowel Cancer Screening Programme. Intern Med J 2016;46:166-71. [Crossref] [PubMed]
- Smith M, Canfell K. Impact of the Australian National Cervical Screening Program in women of different ages. Med J Aust 2016;205:359-64. [Crossref] [PubMed]
- Woods LM, Rachet B, Coleman MP. Origins of socio-economic inequalities in cancer survival: a review. Ann Oncol 2006;17:5-19. [Crossref] [PubMed]
- Booth CM, Li G, Zhang‐Salomons J, et al. The impact of socioeconomic status on stage of cancer at diagnosis and survival. Cancer 2010;116:4160-7. [Crossref] [PubMed]
- Australian Institute of Health Welfare. Cancer Survival and Prevalence in Australia: Period Estimates from 1982 to 2010: AIHW, 2012.
- Stanbury JF, Baade PD, Yu Y, et al. Cancer survival in New South Wales, Australia: socioeconomic disparities remain despite overall improvements. BMC Cancer 2016;16:48. [Crossref] [PubMed]
- Tervonen HE, Aranda S, Roder D, et al. Cancer survival disparities worsening by socio-economic disadvantage over the last 3 decades in new South Wales, Australia. BMC Public Health 2017;17:691. [Crossref] [PubMed]
- Jong KE, Smith DP, Yu XQ, et al. Remoteness of residence and survival from cancer in New South Wales. Med J Aust 2004;180:618-22. [PubMed]
- Yu XQ, Luo Q, Kahn C, et al. Temporal trends show improved breast cancer survival in Australia but widening urban–rural differences. Breast 2015;24:524-7. [Crossref] [PubMed]
- Yu XQ, Luo Q, Smith DP, et al. Geographic variation in prostate cancer survival in New South Wales. Med J Aust 2014;200:586-90. [Crossref] [PubMed]
- Australian Institute of Health and Welfare. National Bowel Cancer Screening Program: monitoring report. 2018. Available online: https://wwwaihwgovau/reports/cancer-screening/national-bowel-cancer-screening-program-2018/contents/summary, accessed 29/5/2018.
- Scollo M, Winstanley M. Tobacco in Australia: facts and issues. Melbourne: Cancer Council Victoria; 2012, accessed on 29/5/2018, editor. 2018.
- US Public Health Service. The health consequences of smoking: a public health service review—1967. US Department of Health, Education, and Welfare, Public Health Service PHS Publication No 1696 1968. Available online: https://profiles.nlm.nih.gov/NN/Views/Exhibit/documents/smoking.html, accessed 29/5/2018.
- Australian Institute of Health and Welfare. National Drug Strategy Household Survey 2016: detailed findings. Drug Statistics series no. 31. Cat. no. PHE 214. AIHW: Canberra, 2017.
- World Health Organisation. Prevention of Noncommunicable Diseases (PND). The WHO Framework Convention on Tobacco Control. Available online: http://www.who.int/tobacco/mpower/publications/brochure_2013/en/, accessed 29/05/2018. 2013.
- Australian Institute of Health and Welfare. Cancer in Australia: Actual incidence data from 1982 to 2013 and mortality data from 1982 to 2014 with projections to 2017. Asia Pac J Clin Oncol 2018;14:5-15. [Crossref] [PubMed]
- Cooper J, Mancuso SG, Borland R, et al. Tobacco smoking among people living with a psychotic illness: the second Australian Survey of Psychosis. Aust N Z J Psychiatry 2012;46:851-63. [Crossref] [PubMed]
- Gibberd A, Supramaniam R, Dillon A, et al. Lung cancer treatment and mortality for Aboriginal people in New South Wales, Australia: results from a population-based record linkage study and medical record audit. BMC Cancer 2016;16:289. [Crossref] [PubMed]
- Australian Government Department of Health. Licensing of tobacco retailers and wholesalers: desirability and best practice arrangements. Available online: http://www.health.gov.au/internet/main/publishing.nsf/Content/tobacco-res-license, accessed 29/05/2018. 2011.
- Wang S, Sun T, Sun H, et al. Survival improvement in patients with non–small cell lung cancer between 1983 and 2012: Analysis of the Surveillance, Epidemiology, and End Results database. Tumour Biol 2017;39:1010428317691677 [PubMed]
- Walters S, Maringe C, Coleman MP, et al. Lung cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK: a population-based study, 2004–2007. Thorax 2013;68:551-64. [Crossref] [PubMed]
- Mitchell ED, Rubin G, Merriman L, et al. The role of primary care in cancer diagnosis via emergency presentation: qualitative synthesis of significant event reports. Br J Cancer 2015;112:S50-6. [Crossref] [PubMed]
- Rankin NM, York S, Stone E, et al. Pathways to lung cancer diagnosis: a qualitative study of patients and general practitioners about diagnostic and pretreatment intervals. Ann Am Thorac Soc 2017;14:742-53. [Crossref] [PubMed]
- Murray SR, Murchie P, Campbell N, et al. Protocol for the CHEST Australia Trial: a phase II randomised controlled trial of an intervention to reduce time-to-consult with symptoms of lung cancer. BMJ Open 2015;5:e008046 [Crossref] [PubMed]
- Rankin NM, Collett GK, Brown CM, et al. Implementation of a lung cancer multidisciplinary team standardised template for reporting to general practitioners: a mixed-method study. BMJ Open 2017;7:e018629 [Crossref] [PubMed]
- Rankin NM, McGregor D, Stone E, et al. Evidence-practice gaps in lung cancer: A scoping review. Eur J Cancer Care (Engl) 2018;27:e12588 [Crossref] [PubMed]
- Whop LJ, Bernardes CM, Kondalsamy-Chennakesavan S, et al. Indigenous Australians with non-small cell lung cancer or cervical cancer receive suboptimal treatment. Asia Pac J Clin Oncol 2017;13:e224-e231. [Crossref] [PubMed]
- Tracey E, McCaughan BC, Young JM, et al. How can we ensure that people with lung cancer living in rural and remote areas are treated surgically when appropriate? Med J Aust 2016;204:330. [Crossref] [PubMed]
- Cramb SM, Garvey G, Valery PC, et al. The first year counts: cancer survival among Indigenous and non-Indigenous Queenslanders, 1997–2006. Med J Aust 2012;196:270-4. [Crossref] [PubMed]
- Coory MD, Green AC, Stirling J, et al. Survival of Indigenous and non-Indigenous Queenslanders after a diagnosis of lung cancer: a matched cohort study. Med J Aust 2008;188:562-6. [PubMed]
- Chynoweth J, Wallington I, Kinsella L, et al. national Aboriginal And Torres Strait Islander Cancer Framework; Directions And Priorities. Asia Pac J Clin Oncol 2015;11:70-1.
- Australian Government Department of Health. The Standing Committee on Screening. Position Statement: Lung Cancer Screening using Low-Dose Computed Tomography, 2015.
- US National Library of Medicine. The International Lung Screen Trial. Available online: https://clinicaltrials.gov/ct2/show/NCT02871856, accessed 29/05/2018. 2016.
- Australian Institute of Health and Welfare. AIHW 2018 Monitoring report. Available online: https://www.aihw.gov.au/reports/cancer-screening/national-bowel-cancer-screening-program-2018/contents/summary, accessed 29/5/2018. 2018.
- Young JP, Win AK, Rosty C, et al. Rising incidence of early‐onset colorectal cancer in Australia over two decades: Report and review. J Gastroenterol Hepatol 2015;30:6-13. [Crossref] [PubMed]
- Whiteman DC, Webb PM, Green AC, et al. Cancers in Australia in 2010 attributable to modifiable factors: summary and conclusions. Aust N Z J Public Health 2015;39:477-84. [Crossref] [PubMed]
- World Cancer Research Fund. Colorectal Cancer report 2017. Available online: https://www.wcrf.org/dietandcancer/colorectal-cancer, accessed 29/5/2018. 2017.
- Mecklin JP. Frequency of hereditary colorectal carcinoma. Gastroenterology 1987;93:1021-5. [Crossref] [PubMed]
- Lynch HT, Lynch PM, Lanspa SJ, et al. Review of the Lynch syndrome: history, molecular genetics, screening, differential diagnosis, and medicolegal ramifications. Clin Genet 2009;76:1-18. [Crossref] [PubMed]
- Keum N, Giovannucci EL. Epidemiology of Colorectal Cancer. Pathology and Epidemiology of Cancer: Springer, 2017:391-407.
- Lew JB, St John DJB, Xu XM, et al. Long-term evaluation of benefits, harms, and cost-effectiveness of the National Bowel Cancer Screening Program in Australia: a modelling study. Lancet Public Health 2017;2:e331-e340. [Crossref] [PubMed]
- Ward PR, Javanparast S, Ah Matt M, et al. Equity of colorectal cancer screening: cross‐sectional analysis of National Bowel Cancer Screening Program data for South Australia. Aust N Z J Public Health 2011;35:61-5. [Crossref] [PubMed]
- Weber MF, Banks E, Smith DP, et al. Cancer screening among migrants in an Australian cohort; cross-sectional analyses from the 45 and Up Study. BMC Public Health 2009;9:144. [Crossref] [PubMed]
- Weber MF, Chiew M, Feletto E, et al. Cancer screening among immigrants living in urban and regional Australia: results from the 45 and up study. Int J Environ Res Public Health 2014;11:8251-66. [Crossref] [PubMed]
- Cancer Institute NSW. Do the test, when it comes in the post. 2018. Available online: https://wwwcancerinstituteorgau/how-we-help/screening-and-early-detection/bowel-screening/do-the-test, accessed 29/5/2018.
- Menzies School of Health Research. National Indigenous Bowel Cancer Screening. 2018. Available online: https://wwwmenzieseduau/page/Research/Indigenous_Health/Cancer/National_Indigenous_Bowel_Cancer_Screening_Program/, accessed 29/5/2018.
- Yu XQ, O'Connell DL, Gibberd RW, et al. A population-based study from New South Wales, Australia 1996-2001: area variation in survival from colorectal cancer. Eur J Cancer 2005;41:2715-21. [Crossref] [PubMed]
- Yu XQ, O'Connell DL, Gibberd RW, et al. Assessing the impact of socio-economic status on cancer survival in New South Wales, Australia 1996-2001. Cancer Causes Control 2008;19:1383-90. [Crossref] [PubMed]
- Weir K, Supramaniam R, Gibberd A, et al. Comparing colorectal cancer treatment and survival for Aboriginal and non-Aboriginal people in New South Wales. Med J Aust 2016;204:156. [Crossref] [PubMed]
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- AIHW & NBOCC. Breast Cancer in Australia: an overview, 2009. Cancer series no. 50. Cat. no. CAN 46. AIHW, Canberra, 2009.
- Burton RC, Bell RJ, Thiagarajah G, et al. Adjuvant therapy, not mammographic screening, accounts for most of the observed breast cancer specific mortality reductions in Australian women since the national screening program began in 1991. Breast Cancer Res Treat 2012;131:949-55. [Crossref] [PubMed]
- Njor SH, Schwartz W, Blichert-Toft M, et al. Decline in breast cancer mortality: how much is attributable to screening? J Med Screen 2015;22:20-7. [Crossref] [PubMed]
- Coleman MP, Forman D, Bryant H, et al. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995–2007 (the International Cancer Benchmarking Partnership): an analysis of population-based cancer registry data. Lancet 2011;377:127-38. [Crossref] [PubMed]
- Dasgupta P, Baade PD, Aitken JF, et al. Multilevel determinants of breast cancer survival: association with geographic remoteness and area-level socioeconomic disadvantage. Breast Cancer Res Treat 2012;132:701-10. [Crossref] [PubMed]
- Cramb SM, Mengersen KL, Baade PD. Spatio-temporal relative survival of breast and colorectal cancer in Queensland, Australia 2001-2011. Spat Spatiotemporal Epidemiol 2016;19:103-14. [Crossref] [PubMed]
- Spillane AJ. What is new in the surgical management and prevention of breast cancer? Med J Aust 2016;204:311-4. [Crossref] [PubMed]
- Giles G, Thursfield V. Prostate Cancer. Canstat 30. Carlton: Anti-Cancer Council of Victoria, Australia, 2000.
- Smith DP, Supramaniam R, Marshall VR, et al. Prostate cancer and prostate-specific antigen testing in New South Wales. Med J Aust 2008;189:315-8. [PubMed]
- Etzioni R, Gulati R. Recent trends in PSA testing and prostate cancer incidence: a look at context. JAMA Oncol 2016;2:955-6. [Crossref] [PubMed]
- Feletto E, Bang A, Cole-Clark D, et al. An examination of prostate cancer trends in Australia, England, Canada and USA: Is the Australian death rate too high? World J Urol 2015;33:1677-87. [Crossref] [PubMed]
- Baade PD, Youlden DR, Coory MD, et al. Urban-rural differences in prostate cancer outcomes in Australia: what has changed. Med J Aust 2011;194:293-6. [PubMed]
- Ruseckaite R, Sampurno F, Millar J, et al. Diagnostic and treatment factors associated with poor survival from prostate cancer are differentially distributed between regional and metropolitan Victoria, Australia. BMC Urol 2016;16:54. [Crossref] [PubMed]
- Smith M, Canfell K. Impact of the Australian National Cervical Screening Program in women of different ages. Med J Aust 2016;205:359-64. [Crossref] [PubMed]
- Simonella L, Canfell K. The impact of a two- versus three-yearly cervical screening interval recommendation on cervical cancer incidence and mortality: an analysis of trends in Australia, New Zealand, and England. Cancer Causes Control 2013;24:1727-36. [Crossref] [PubMed]
- Australian Institute of Health and Welfare. Cervical screening in Australia 2014–2015. Cancer series no. 105. Cat. no. CAN 104. Canberra: AIHW, 2017.
- National HPV Vaccination Program Register. HPV vaccination coverage data 2017 [updated October 2017. Available online: http://www.hpvregister.org.au/research/coverage-data, accessed 29/5/2018.
- Brisson M, Bénard É, Drolet M, et al. Population-level impact, herd immunity, and elimination after human papillomavirus vaccination: a systematic review and meta-analysis of predictions from transmission-dynamic models. Lancet Public Health 2016;1:e8-e17. [Crossref] [PubMed]
- Garland SM, Kjaer SK, Munoz N, et al. Impact and Effectiveness of the Quadrivalent Human Papillomavirus Vaccine: A Systematic Review of 10 Years of Real-world Experience. Clin Infect Dis 2016;63:519-27. [Crossref] [PubMed]
- Machalek DA, Garland SM, Brotherton JML, et al. Very low prevalence of vaccine human papillomavirus (HPV) types among 18 to 35 year old Australian women, nine years following implementation of vaccination. J Infect Dis 2018;217:1590-600. [Crossref] [PubMed]
- Brotherton JML, Tabrizi SN, Phillips S, et al. Looking beyond human papillomavirus (HPV) genotype 16 and 18: defining HPV genotype distribution in cervical cancers in Australia prior to vaccination. Int J Cancer 2017;141:1576-84. [Crossref] [PubMed]
- Office of the Prime Minister of Australia. A new vaccine to strengthen the health of young Australians 2017 [updated 8th October Available online: https://www.pm.gov.au/media/2017-10-08/new-vaccine-strengthen-health-young-australians, accessed 29/5/2018.
- Whop LJ, Garvey G, Baade P, et al. The first comprehensive report on Indigenous Australian women's inequalities in cervical screening: A retrospective registry cohort study in Queensland, Australia (2000–2011). Cancer 2016;122:1560-9. [Crossref] [PubMed]
- Smith MA, Liu B, McIntyre P, et al. Trends in genital warts by socioeconomic status after the introduction of the national HPV vaccination program in Australia: analysis of national hospital data. BMC Infect Dis 2016;16:52. [Crossref] [PubMed]
- Smith MA, Liu B, McIntyre P, et al. Fall in genital warts diagnoses in the general and Indigenous Australian population following a national HPV vaccination program: analysis of routinely collected national hospital data. J Infect Dis 2015;211:91-9. [Crossref] [PubMed]
- Ali H, McManus H, O'Connor CC, et al. Human papillomavirus vaccination and genital warts in young Indigenous Australians: national sentinel surveillance data. Med J Aust 2017;206:204-9. [Crossref] [PubMed]
- Cancer Council Australia Cervical Cancer Screening Guidelines Working Party. National Cervical Screening Program: Guidelines for the management of screen-detected abnormalities, screening in specific populations and investigation of abnormal vaginal bleeding 2017. Available online: http://wiki.cancer.org.au/australia/Guidelines:Cervical_cancer/Screening, accessed 29/5/2018.
- Medical Services Advisory Committee. National Cervical Screening Program Renewal: Evidence Review (Assessment Report). MSAC Application No. 1276. Canberra: Australian Government Department of Health 2013.
- Lew JB, Simms K, Smith MA, et al. National Cervical Screening Program Renewal: Effectiveness modelling and economic evaluation in the Australian setting (Assessment Report). MSAC application number 1276. Canberra: Department of Health, 2014.
- Lew JB, Simms K, Smith MA, et al. Primary HPV testing versus cytology-based cervical screening in women in Australia vaccinated for HPV and unvaccinated: effectiveness and economic assessment for the National Cervical Screening Program. Lancet Public Health 2017;2:e96-e107. [Crossref] [PubMed]
- Verdoodt F, Jentschke M, Hillemanns P, et al. Reaching women who do not participate in the regular cervical cancer screening programme by offering self-sampling kits: a systematic review and meta-analysis of randomised trials. Eur J Cancer 2015;51:2375-85. [Crossref] [PubMed]
- Sultana F, English DR, Simpson JA, et al. Home-based HPV self-sampling improves participation by never- and under-screened women: Results from a large randomised trial (iPap) in Australia. Int J Cancer 2016;139:281-90. [Crossref] [PubMed]
- McLachlan E, Anderson S, Hawkes D, et al. Completing the cervical screening pathway: Factors that facilitate the increase of self-collection uptake among under-screened and never-screened women, an Australian pilot study. Curr Oncol 2018;25:e17-26. [Crossref] [PubMed]
- Smith M, Lew JB, Simms K, et al. Impact of HPV sample self-collection for underscreened women in the renewed Cervical Screening Program. Med J Aust 2016;204:1941e-7. [Crossref] [PubMed]
- Simms KT, Smith MA, Lew JB, et al. Will cervical screening remain cost-effective in women offered the next generation nonavalent HPV vaccine? Results for four developed countries. Int J Cancer 2016;139:2771-80. [Crossref] [PubMed]
- Kim JJ, Burger EA, Sy S, et al. Optimal Cervical Cancer Screening in Women Vaccinated Against Human Papillomavirus. J Natl Cancer Inst 2016;109:djw216 [Crossref] [PubMed]
- Pedersen K, Burger EA, Nygard M, et al. Adapting cervical cancer screening for women vaccinated against human papillomavirus infections: The value of stratifying guidelines. Eur J Cancer 2018;91:68-75. [Crossref] [PubMed]
- Forman D, Bray F, Brewster D. Cancer in five continents: volume X. Lyon, France: International Agency for Research on Cancer, 2014.
- Whiteman DC, Bray CA, Siskind V, et al. A comparison of the anatomic distribution of cutaneous melanoma in two populations with different levels of sunlight: the west of Scotland and Queensland, Australia 1982–2001. Cancer Causes Control 2007;18:485-91. [Crossref] [PubMed]
- LaVeist T, Gaskin D, Richard P. The economic burden of health inequalities in the United States. 2009. Joint Center for Political and Economic Studies Available online: http://www.jointcenter.org, accessed 29/5/2018.
Cite this article as: Cheng ES, Weber M, Feletto E, Smith MA, Yu XQ. Cancer burden and control in Australia: lessons learnt and challenges remaining. Ann Cancer Epidemiol 2018;2:3.


