Cancer burden in Japan based on the latest cancer statistics: need for evidence-based cancer control programs
Introduction
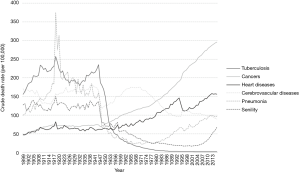
Cancer as a cause of death has been constantly increasing in Japan, and it became the leading cause of death in 1981 (Figure 1). Japan had a baby boomer generation, namely those born in 1947–1948 just after World War II, as well as a second generation of baby boomers. The first generation of baby boomers is now in their mid-70s, an age when cancers occur frequently. Accordingly, the number of cancer patients is increasing, and the cancer burden on society is becoming increasingly heavier.
The first population-based cancer registry (PBCR) started in Miyagi Prefecture in 1951. However, data were collected and data quality was evaluated only for several selected regions since the 1970s (1). The Cancer Control Act, approved in 2006, mentioned that the PBCR system needed to be developed to determine cancer incidence throughout Japan. Development of hospital-based cancer registries (HBCRs, in the 434 designated cancer treating hospitals, as of 2018) has clearly supported infrastructure development of cancer registries and led to improvement in the completeness of data after 2010 (Table 1). The Act on Promoting Cancer Registries was finally enacted in Japan on December 6th, 2013. This Act provides for the implementation of a National Cancer Registry in accordance with the purpose of the Cancer Control Act (2,3). The National Cancer Registry provides accurate and immediate cancer statistics. According to the Act, hospitals report to the prefectural governors information on any primary cancer that was first diagnosed from January 1st, 2016, when cancer became a reportable disease.
Table 1
| Year of incidence | DCN% | DCO% | M/I | MV% |
|---|---|---|---|---|
| 2003 | 22.8 | 16.8 | 0.50 | 68.1 |
| 2004 | 23.6 | 17.1 | 0.51 | 67.2 |
| 2005 | 20.1 | 14.9 | 0.50 | 74.9 |
| 2006 | 20.8 | 13.4 | 0.49 | 74.2 |
| 2007 | 20.6 | 14.6 | 0.49 | 74.3 |
| 2008 | 20.2 | 13.6 | 0.47 | 75.5 |
| 2009 | 20.1 | 13.4 | 0.45 | 76.0 |
| 2010 | 18.1 | 11.8 | 0.45 | 78.0 |
| 2011 | 11.9 | 5.3 | 0.43 | 83.1 |
| 2012 | 13.1 | 5.6 | 0.43 | 82.2 |
| 2013 | 8.3 | 5.0 | 0.43 | 83.7 |
| 2014 | 9.1 | 6.4 | 0.42 | 82.3 |
PBCR, population-based cancer registry; DCN%, proportion of death certificate notification cases; DCO%, proportion of death certificate only cases; M/I, ratio of mortality to incidence; MV%, proportion of microscopically verified cases.
The purpose of this article was to determine and summarize the current status of cancer burden in this hyper-aging country using up-to-date cancer statistics. A further aim was to introduce the major change in cancer registry according to the Act on Promoting Cancer Registries.
Methods
Vital statistics in Japan are used for population prediction, and cancer mortality statistics as a reliable data source since 1898 provide further relevant information. Yearly publication of mortality data in Japan is done in the following year. According to the death certificates issued by medical doctors, the city halls publish a form for vital statistics. The Ministry of Health, Labour and Welfare (MHLW) collects all the forms from the city halls via the prefectures, and then the causes of death are coded using ICD-10. The ministry uses the world standard format for death certificates and follows the rules for selection of cause of death. The data for the year 2014 were obtained from the ministry website and used for this article in accordance with the incidence date, but the latest data are those for the year 2016. Almost all aggregated mortality data are publicly available online (4).
Availability of incidence data varies according to prefecture. In several prefectures, such as Miyagi, Fukui, and Osaka, the data are available from the 1970s. Half of the prefectures have data since the early 1990s, and the other half have data since the late 2000s. Data for all 47 prefectures exist since 2013, and the latest fixed data as of July 2018 are for the year 2014. In 2005, the Japan Cancer Surveillance Research Group (JCSRG), which was initiated under the Third Ten-year Comprehensive Strategy for Cancer Control Program [2004–2013], took over the research group in Osaka to perform national estimates of cancer incidence from 2000 (5), which is called the Monitoring of Cancer Incidence in Japan (MCIJ) project. The data for the year 2014 were collected from all 47 prefectures in 2017 as part of the MCIJ project, and national incidence was calculated.
Population estimates are done by the Statistics Bureau by interpolating two national census populations in 2010 and 2015. Populations are also publicly available in the website (4). Both the mortality and incidence rates were calculated using the estimated population, and age-standardized to world population.
Since there is a lack of long-term high quality cancer registry data, the data of three prefectures, Miyagi, Fukui, and Nagasaki, which represent around 4% of the total population, were used.
Results
Cancer mortality in 2014
The mortality, crude rates, and age-standardized rates in 2014 are presented in Table 2. The total cancer mortality in Japan in 2014 was 368,103 (C00–C96), including 218,397 males and 149,706 females. The age-standardized mortality rates (world population) for males and females were 114.8 and 63.0/100,000, respectively. Among males, in 2014, the leading cancer site was the lung (24.0%), followed by the stomach (14.4%), colon and rectum (12.0%), liver (8.8%), and pancreas (7.5%). In 2014, the leading cancer site in females was the colon and rectum (14.9%), followed by the lung (14.0%), stomach (11.0%), pancreas (10.0%), and breast (8.8%).
Table 2
| Primary sites | ICD10 | Mortality | Crude rate*1 | ASR (world population)*1 |
|---|---|---|---|---|
| Male | ||||
| All sites | C00–C96 | 218,397 | 357.8 | 114.8 |
| Lip, oral cavity, and pharynx | C00–C14 | 5,268 | 8.6 | 3.1 |
| Esophagus | C15 | 9,629 | 15.8 | 5.6 |
| Stomach | C16 | 31,483 | 51.6 | 16.2 |
| Colon and rectum | C18–C20 | 26,177 | 42.9 | 14.5 |
| Colon | C18 | 16,478 | 27.0 | 8.7 |
| Rectum | C19–C20 | 9,699 | 15.9 | 5.8 |
| Liver | C22 | 19,208 | 31.5 | 10.1 |
| Gallbladder, etc. | C23–C24 | 9,052 | 14.8 | 4.2 |
| Pancreas | C25 | 16,411 | 26.9 | 9.2 |
| Larynx | C32 | 908 | 1.5 | 0.5 |
| Trachea, bronchus, and lung | C33–C34 | 52,505 | 86.0 | 26.6 |
| Skin, including melanoma | C43–C44 | 797 | 1.3 | 0.4 |
| Prostate | C61 | 11,507 | 18.9 | 4.6 |
| Bladder | C67 | 5,308 | 8.7 | 2.4 |
| Kidney, renal pelvis, ureter, etc. | C64–C66, C68 | 5,721 | 9.4 | 3.0 |
| Brain and nervous system | C70–C72 | 1,329 | 2.2 | 1.2 |
| Thyroid | C73 | 570 | 0.9 | 0.3 |
| Malignant lymphoma | C81–C85, C96 | 6,457 | 10.6 | 3.3 |
| Multiple myeloma | C88, C90 | 2,203 | 3.6 | 1.1 |
| All leukemias | C91–C95 | 4,896 | 8.0 | 3.2 |
| Female | ||||
| All sites | C00–C96 | 149,706 | 232.5 | 63.0 |
| Lip, oral cavity, and pharynx | C00–C14 | 2,147 | 3.3 | 0.8 |
| Esophagus | C15 | 1,947 | 3.0 | 0.9 |
| Stomach | C16 | 16,420 | 25.5 | 6.2 |
| Colon and rectum | C18–C20 | 22,308 | 34.6 | 8.5 |
| Colon | C18 | 16,819 | 26.1 | 6.1 |
| Rectum | C19–C20 | 5,489 | 8.5 | 2.5 |
| Liver | C22 | 10,335 | 16.1 | 3.2 |
| Gallbladder, etc. | C23–C24 | 9,065 | 14.1 | 2.7 |
| Pancreas | C25 | 15,305 | 23.8 | 5.8 |
| Larynx | C32 | 70 | 0.1 | 0.0 |
| Trachea, bronchus, and lung | C33–C34 | 20,891 | 32.4 | 7.7 |
| Skin, including melanoma | C43–C44 | 860 | 1.3 | 0.3 |
| Breast | C50 | 13,240 | 20.6 | 8.9 |
| Uterus | C53–C55 | 6,429 | 10.0 | 4.3 |
| Cervix uteri | C53 | 2,902 | 4.5 | 2.2 |
| Corpus uteri | C54 | 2,243 | 3.5 | 1.4 |
| Ovary | C56 | 4,840 | 7.5 | 3.1 |
| Bladder | C67 | 2,452 | 3.8 | 0.7 |
| Kidney, renal pelvis, ureter, etc. | C64–C66, C68 | 3,072 | 4.8 | 1.0 |
| Brain and nervous system | C70–C72 | 973 | 1.5 | 0.8 |
| Thyroid | C73 | 1,192 | 1.9 | 0.4 |
| Malignant lymphoma | C81–85, C96 | 5,075 | 7.9 | 1.8 |
| Multiple myeloma | C88, C90 | 1,982 | 3.1 | 0.7 |
| All leukemias | C91–C95 | 3,300 | 5.1 | 1.7 |
*1, per 100,000 population. ICD-10, International Classification of Disease, 10th Revision.
Cancer incidence in 2014
The total cancer incidence in Japan in 2014 was estimated to be 867,408 (C00–C96), with 501,527 males and 365,881 females affected (Table 3) (6). The age-standardized incidence rates (world population) for males and females were 303.1 and 224.8 /100,000, respectively. Regarding the quality and completeness of reporting, the overall proportion of death certificate-only cases in all cancer cases (DCO%) and the ratio of mortality to incidence (M/I) were 6.4% and 0.42, respectively. For accuracy of diagnosis, the overall ratio of morphologically verified diagnosis (cases diagnosed based on histological and cytological examination) in all cancer cases (MV%) was 82.3%. Among males, the leading cancer site was the stomach (17.3%), followed by the lung (15.3%), colon and rectum (15.3%), and prostate (14.7%). In 2014, the leading cancer site in females was the breast (20.8%), followed by the colon and rectum (15.8%) and stomach (10.8%). The five leading primary sites accounted for 68.0% of the total incidence in males and 64.0% in females.
Table 3
| Primary site | ICD10 | Incidence | Crude rate*1 | ASR (world population)*1 | DCO/I (%) | M/I | MV/I (%) |
|---|---|---|---|---|---|---|---|
| Male | |||||||
| All sites | C00–C96 | 501,527 | 810.6 | 303.1 | 4.8 | 0.43 | 82.2 |
| Lip, oral cavity, and pharynx | C00–C14 | 13,378 | 21.6 | 9.4 | 3.2 | 0.39 | 90.4 |
| Esophagus | C15 | 19,067 | 30.8 | 11.9 | 3.8 | 0.51 | 90.3 |
| Stomach | C16 | 86,656 | 140.1 | 49.6 | 4.2 | 0.36 | 91.1 |
| Colon and rectum | C18–C20 | 76,718 | 124.0 | 48.3 | 4.2 | 0.34 | 89.6 |
| Colon | C18 | 47,275 | 76.4 | 28.1 | 4.6 | 0.35 | 88.4 |
| Rectum | C19–C20 | 29,443 | 47.6 | 20.2 | 3.6 | 0.33 | 91.4 |
| Liver | C22 | 27,315 | 44.1 | 15.9 | 8.7 | 0.70 | 32.3 |
| Gallbladder, etc. | C23–C24 | 11,641 | 18.8 | 5.9 | 7.3 | 0.78 | 64.7 |
| Pancreas | C25 | 18,745 | 30.3 | 11.1 | 6.7 | 0.88 | 55.1 |
| Larynx | C32 | 4,798 | 7.8 | 2.9 | 2.7 | 0.19 | 91.6 |
| Trachea, bronchus, and lung | C33–C34 | 76,879 | 124.3 | 42.6 | 6.4 | 0.68 | 76.9 |
| Skin, including melanoma | C43–C44 | 9,871 | 16.0 | 5.6 | 0.6 | 0.08 | 96.3 |
| Prostate | C61 | 73,764 | 119.2 | 39.7 | 3.7 | 0.16 | 87.7 |
| Bladder | C67 | 15,486 | 25.0 | 8.3 | 4.7 | 0.34 | 87.3 |
| Kidney, renal pelvis, ureter, etc. | C64–C66, C68 | 16,781 | 27.1 | 11.2 | 3.6 | 0.34 | 81.6 |
| Brain and nervous system | C70–C72 | 3,042 | 4.9 | 3.1 | 7.2 | 0.44 | 70.6 |
| Thyroid | C73 | 3,788 | 6.1 | 3.4 | 2.1 | 0.15 | 93.2 |
| Malignant lymphoma | C81–C85, C96 | 15,733 | 25.4 | 11.0 | 3.3 | 0.41 | 91.4 |
| Multiple myeloma | C88, C90 | 3,582 | 5.8 | 2.1 | 8.6 | 0.61 | 80.8 |
| All leukemias | C91–C95 | 7,227 | 11.7 | 7.0 | 0.9 | 0.68 | 98.2 |
| Female | |||||||
| All sites | C00–C96 | 365,881 | 558.0 | 224.8 | 6.2 | 0.41 | 81.1 |
| Lip, oral cavity, and pharynx | C00–C14 | 5,494 | 8.4 | 3.2 | 4.6 | 0.39 | 87.7 |
| Esophagus | C15 | 3,643 | 5.6 | 2.0 | 6.0 | 0.53 | 85.7 |
| Stomach | C16 | 39,493 | 60.2 | 18.1 | 7.1 | 0.41 | 87.0 |
| Colon and rectum | C18–C20 | 57,735 | 88.0 | 28.9 | 6.7 | 0.39 | 84.5 |
| Colon | C18 | 41,228 | 62.9 | 19.2 | 7.3 | 0.41 | 82.8 |
| Rectum | C19–C20 | 16,507 | 25.2 | 9.7 | 5.1 | 0.33 | 89.0 |
| Liver | C22 | 13,512 | 20.6 | 5.2 | 13.5 | 0.76 | 24.7 |
| Gallbladder, etc. | C23–C24 | 10,699 | 16.3 | 3.6 | 12.0 | 0.85 | 52.4 |
| Pancreas | C25 | 17,411 | 26.6 | 7.1 | 10.0 | 0.88 | 47.1 |
| Larynx | C32 | 357 | 0.5 | 0.2 | 2.3 | 0.20 | 87.5 |
| Trachea, bronchus, and lung | C33–C34 | 35,739 | 54.5 | 16.7 | 8.3 | 0.58 | 76.0 |
| Skin, including melanoma | C43–C44 | 9,657 | 14.7 | 4.0 | 1.1 | 0.09 | 95.3 |
| Breast | C50 | 76,257 | 116.3 | 64.0 | 3.0 | 0.17 | 93.1 |
| Uterus | C53–C55 | 24,944 | 38.0 | 22.9 | 3.0 | 0.26 | 92.7 |
| Cervix uteri | C53 | 10,490 | 16.0 | 10.5 | 2.1 | 0.28 | 94.1 |
| Corpus uteri | C54 | 13,889 | 21.2 | 12.2 | 1.5 | 0.16 | 94.6 |
| Ovary | C56 | 10,011 | 15.3 | 8.9 | 6.1 | 0.48 | 84.1 |
| Bladder | C67 | 5,109 | 7.8 | 1.9 | 9.2 | 0.48 | 79.1 |
| Kidney, renal pelvis, ureter, etc. | C64–C66, C68 | 7,900 | 12.0 | 4.1 | 5.6 | 0.39 | 75.3 |
| Brain and nervous system | C70–C72 | 2,669 | 4.1 | 2.3 | 10.8 | 0.36 | 61.9 |
| Thyroid | C73 | 10,564 | 16.1 | 9.7 | 2.5 | 0.11 | 93.0 |
| Malignant lymphoma | C81–C85, C96 | 13,635 | 20.8 | 8.1 | 4.3 | 0.37 | 90.1 |
| Multiple myeloma | C88, C90 | 3,119 | 4.8 | 1.4 | 8.9 | 0.64 | 80.1 |
| All leukemias | C91–C95 | 4,967 | 7.6 | 4.4 | 1.2 | 0.66 | 97.0 |
*1, per 100 000 population. ICD-10, International Classification of Disease, 10th Revision; DCO/I, proportion of cases with the death certificate only to incident cases; M/I, number of deaths/number of incidence; MV/I, proportion of microscopically verified cases to incident cases.
Compared to other cancers, in both males and females, pancreatic and lung cancers were generally more likely to be diagnosed after distant metastasis had occurred. For skin, including melanoma, localized cancer accounted for a large part of the extent of disease at diagnosis (83.8%). The proportion was comparatively higher in each of laryngeal (68.8%), bladder (65.8%), and uterine body (65.7%) cancers. On the other hand, the proportion was low in pancreas (7.4%), ovary (23.1%), and lung (31.7%) cancers. Prostate, breast, and cervical cancers had a relatively high proportion detected by cancer screening (23.2%, 21.6%, and 16.8%, respectively) according to the cancer case reports in the cancer registry.
Childhood cancer and adolescent and young adulthood (AYA) cancer are also topics to be considered (7). Cancers diagnosed at 0–39 years of age were classified according to the International Classification of Childhood Cancer, version 3. The national estimates of cancer incidence were 2,055 for 0–14 years (1,118 males and 937 females), 864 for 15–19 years (450 males and 414 females), 4246 for 20–29 years (1,699 males and 2,547 females), and 16,295 for 30–39 years of age (5,101 males and 11,194 females). The five leading cancers were leukemia, cancer of the central nervous system, lymphoma, malignant germ cell and other gonadal tumors, and neuroblastoma in childhood cases (0–14 years old). Breast and cervix uteri were the most frequent sites of cancers for the age group of 30–39 years, with an increased incidence in females in this age group. Five PBCRs participated in the latest international incidence of childhood cancer study organized by International Agency for Research on Cancer (IARC) (8), and they showed a lower age-standardized incidence rate compared with the USA and Europe, but the gap was much smaller than in adults. Rare cancer research was done before (9), showing that the estimated incidence of rare cancers was about 75 per 100,000 between 1998 and 2007, which corresponds to about 94,800 new diagnoses in 2012 or approximately 15% of all cancer diagnoses.
Risk factors for cancer in Japan have been routinely examined in studies with Japanese participants since 2005 (10). Meta-analyses are done by risk factors and primary sites, and the results are updated on the website of the National Cancer Center, Japan. Preventable attributable risk is also calculated based on the results of cohort studies. According to the results, 55% of cancers among males and about 30% among females had preventable factors (11). In males, smoking was the most important risk factor (30% for incidence and 35% for mortality), followed by infectious agents (23% and 23%). In females, in contrast, infectious agents, mainly HPV, had the highest effect (18% and 19%). Cancer prevention programs are promoted in the prefectures based on such evidence (12).
Cancer survival in Japan
The first collaborative study on cancer survival was organized by a research group in Osaka in the early 2000s (13), and the JCSRG took over their role (14). Recently, long-term survival and conditional survival, partly by the period method, were also estimated based on various PBCR data (15). As of 2016, 27 of 47 prefectures are able to follow-up all patients and to calculate 5-year survival rates for the patients diagnosed in 2006–2008 (16). CONCORD-3 is one of the international collaborative studies on cancer survival based on individual treatment data from 71 countries, equivalent to 67% of the world population, with 322 population-based cancer registries in the survival rate study of 37,500,000 cases diagnosed in the years 2000–2014 (17). From Japan, 16 PBCRs participated in the study. According to the study results, the areas with the highest cancer survival rates were North America, Oceania, and northern Europe. Japan also showed good results.
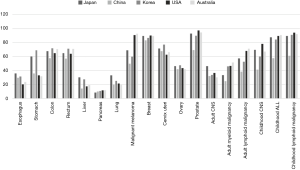
Though 5-year net survival of gastric cancer is around 20–40% in other countries, the survival rate is high, over 60%, in Japan and Korea (Figure 2). Other specific primary sites include liver cancer in Japan. Survivals in Korea, Singapore, and Taiwan were more than 20%, which was seen in only five countries, with survivals of 5–30% in many countries. Japan was higher than 30%, and 5–10% improvement was found for such intractable cancers in Japan. The survival rate was globally still low in lung cancer, around 20% in Europe, but 39% in Japan.
On the other hand, the prognosis of skin malignant melanoma is very poor in Asia. The survival rate of adult myeloid and lymphatic disease was 30–50% globally, but it was low in Asian countries, with a rate of 33.3% in Japan. Survival for childhood acute lymphocytic leukemia and childhood lymphoma was less than 90% in Japan, while it was more than 90% in other developed countries.
Cancer screening is promoted to decrease cancer incidence, improve survival, and decrease mortality. In Japan, the national committee currently appraises evidence for cancer screening and publishes guidelines (18), and it recommends cancer screening for stomach, colorectal, lung, breast, and cervical cancers. Recently, the screening methods for breast and gastric cancers have been revised based on the cancer screening guidelines. However, not all local governments follow the guidelines, and the importance of promoting evidence-based cancer screening for national programs is increasingly being recognized. In addition, cancer screening is not regularly evaluated in terms of sensitivity and specificity. Only in a few prefectures are such activities performed, but as part of research projects (19).
Cancer burden in Japan according to mortality, incidence, and survival
Geographical pattern
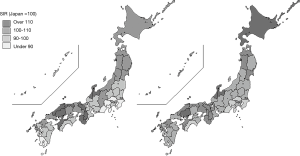
In Japan, there are 47 prefectures that function as administrative units. The largest prefecture is Tokyo, with 12.6 million people, and the smallest is Tottori prefecture, with 0.6 million, so there is a large variation in population. Because of the territories’ wide range of latitude, seasonal winds, and different types of ocean currents, Japan has a variety of climates and cultures that shape people’s lifestyles. There were clear regional disparities in incidences for some primary sites, such as stomach, liver, lung, and leukemia, but not for others, such as colon and rectum (Figure 3). On the whole, cancer incidence is high in areas along the Sea of Japan. Liver cancer occurs often in the west of Japan, especially in Kinki (west) and Kyushu (south west) areas, and a high incidence of leukemia is evident in Kyushu. Lung cancer is frequent in Hokkaido (north) and in the Kyushu area. The gaps between standardized incidence ratio (SIR) and standardized mortality ratio (SMR) for all primary sites varied according to primary sites and prefectures (Table 4). In Aomori, Akita, Tokyo, Wakayama, and Fukuoka, cancer risks, both incidence and mortality, were higher than in the other prefectures, and in Fukui, Shiga, Kochi, and Okinawa, the risks were comparatively low. In Nagano, despite a higher incidence, the risk of dying from cancer was the lowest in Japan.
Table 4
| SMR | SIR | ||||
|---|---|---|---|---|---|
| <90 | 90–100 | N.S. | 100–110 | >110 | |
| Male | |||||
| >110 | 2 Aomori | ||||
| 100–110 | 35 Yamaguchi; 41 Saga |
1 Hokkaido; 27 Osaka; 28 Hyogo; 40 Fukuoka; 42 Nagasaki |
5 Akita; 30 Wakayama; 31 Tottori |
||
| N.S. | 46 Kagoshima | 3 Iwate; 7 Fukushima; 8 Ibaraki; 9 Tochigi |
15 Niigata | 4 Miyagi; 6 Yamagata; 13 Tokyo; 16 Toyama; 26 Kyoto; 29 Nara; 32 Shimane; 38 Ehime |
17 Ishikawa; 34 Hiroshima |
| 90–100 | 12 Chiba; 14 Kanagawa; 19 Yamanashi; 36 Tokushima; 47 Okinawa |
10 Gunma; 11 Saitama; 22 Shizuoka; 23 Aichi; 24 Mie; 25 Shiga; 39 Kochi; 43 Kumamoto; 44 Oita |
21 Gifu; 37 Kagawa |
33 Okayama; 45 Miyazaki |
|
| <90 | 18 Fukui | 20 Nagano | |||
| Female | |||||
| >110 | 2 Aomori | ||||
| 100–110 | 28 Hyogo | 42 Nagasaki | 1 Hokkaido; 26 Kyoto; 27 Osaka; 40 Fukuoka |
13 Tokyo | |
| N.S. | 19 Yamanashi | 8 Ibaraki; 9 Tochigi; 10 Gunma; 11 Saitama; 14 Kanagawa; 15 Niigata; 23 Aichi; 41 Saga |
4 Miyagi; 6 Yamagata; 18 Fukui; 29 Nara |
5 Akita; 16 Toyama; 21 Gifu; 30 Wakayama; 31 Tottori |
17 Ishikawa |
| 90–100 | 12 Chiba; 36 Tokushima; 39 Kochi; 46 Kagoshima |
3 Iwate; 7 Fukushima; 22 Shizuoka; 25 Shiga; 35 Yamaguchi; 43 Kumamoto; 44 Oita; 47 Okinawa |
24 Mie; 32 Shimane; 33 Okayama; 37 Kagawa; 38 Ehime |
45 Miyazaki | 34 Hiroshima |
| <90 | 20 Nagano | ||||
Summary of trends
Trends of cancer incidence and mortality are examined by using three high-quality PBCR data sets (20). Since the quality of cancer registry data has improved over a long time, it leads to misunderstanding if we use all of the PBCR data. Looking at the age-standardized mortality rate for all cancer sites combined, the trends are very different from the crude rates in Figure 1. For males, there was a slight increase up to 1990, with a decrease since then. For females, the rate has been steadily decreasing. This indicates that the steep increase in the number of deaths from cancer is completely due to the growing number of elderly persons in Japan. Table 5 shows the recent trends in cancer mortality and incidence rates adjusted by the world standard population using joinpoint analysis. No increase of mortality was observed in any primary sites in males. However, in females, uterine cancer, both of the cervix and the body, showed increasing mortality. Mortality in all the other primary sites is decreasing or at least leveling off. As for incidence, pancreas, prostate, and thyroid cancers and malignant lymphoma were increasing in males, and esophagus, colon and rectum, lung, breast, uterus, ovary, and thyroid cancers and malignant lymphoma were increasing in females. After the Great East Japan Earthquake, there was concern about an increase of cancer incidence in several primary sites, such as the thyroid and leukemia. Changes of trends in incidence were found for the colorectum and thyroid in females (21).
Table 5
| Primary site | ICD-10th | Incidence | Survival (1993–2014) | Mortality |
|---|---|---|---|---|
| Male | ||||
| Esophagus | C15 | + | + | − |
| Stomach | C16 | = | + | − |
| Colon and rectum | C18–C20 | = | + | = |
| Colon | C18 | = | + | = |
| Rectum | C19–C20 | = | + | − |
| Liver | C22 | - | + | − |
| Gallbladder, etc. | C23–C24 | - | − | |
| Pancreas | C25 | + | = | = |
| Trachea, bronchus, and lung | C33–C34 | = | + | − |
| Prostate | C61 | + | + | − |
| Malignant lymphoma | C81–C85, C96 | + | + | = |
| All leukemias | C91–C95 | = | + | − |
| Female | ||||
| Esophagus | C15 | + | + | − |
| Stomach | C16 | − | + | − |
| Colon and rectum | C18–C20 | = | + | = |
| Colon | C18 | + | + | = |
| Rectum | C19–C20 | + | + | − |
| Liver | C22 | − | + | − |
| Pancreas | C25 | = | = | = |
| Trachea, bronchus, and lung | C33–C34 | + | + | − |
| Breast | C50 | + | = | = |
| Uterus | C53–C55 | + | + | + |
| Ovary | C56 | + | + | − |
| Malignant lymphoma | C81–C85, C96 | + | + | = |
| All leukemias | C91–C95 | = | + | − |
+, increasing; =, levelling off; −, decreasing.
Prevalence and long-term prediction
The national estimated cancer incidence for the year 2013 and survival from 2006–2008 were used to calculate 5-year prevalence. The future population (2015–2039) was estimated by the National Institute of Population and Social Security Research. Cancer incidence during the period 1980–2006 was used to estimate age-specific incidence rates by sex, 6 time periods (80–84, 85–89, 90–94, 95–99, 00–04, 05–06), 25 primary sites, and 18 age groups. Prediction of cancer incidence to the year 2035 was done according to a Poisson regression model, by age, period, and cohort, using the method of NORDCAN.
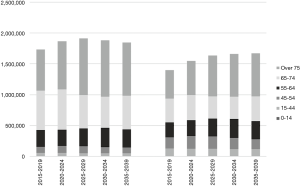
The 5-year prevalence for all cancers (C00–C96) was calculated according to the methods of Pisani (22), as 1,734,060, corresponding to 2.8% of the population in males and 1,399,380 (2.2%) in females (Figure 4). The prevalence was highest for the prostate (26.0% of all cancer sites) in males, followed by stomach (17.7%), and colon and rectum (17.1%). Breast was the leading site in females (29.6%), followed by colon and rectum (15.4%) and stomach (9.7%). The prevalence in males increases steadily to the period 2025–2029, and then it is expected to take a downward turn. The prevalence in females continues to increase through the estimation period, but the increase slows after the period 2025–2029. For all cancers combined, the proportions of elderly cancer patients (75 years and over) were 38.5% in males and 33.3% in females in 2015–2019, and then exceeded 40% for both sexes.
Discussion
In 2006, the Cancer Control Act mentioned the necessity of developing a system for determining the national incidence of cancer. The JCSRG moved toward standardization of registration methods. As a result, in Cancer in Five Continents, volume XI, 9 registries from Japan submitted the data, and all of them were accepted (23). PBCRs in Japan have a long history, but the situation was clearly different with the very complete mortality data provided promptly by the national vital statistics. More than two-thirds of the 47 prefectures started such registries before the early 1990s. The number of prefectures with PBCRs has gradually increased during the Third Ten-year Comprehensive Strategy for Cancer Control Program: 32 in 2004, 35 in 2009, 45 in 2011, and 47 in 2013. Thus, the prefectures have now recognized the importance of the cancer registration system.
Nonetheless, until the MCIJ project, we had no experience collecting incidence data from all prefectures conducting cancer registration. The MCIJ was the first step in achieving “national” figures, as outlined in the Act. Timeliness is a critical issue. Prefectures starting cancer registration require about 3 to 4 years to fix the statistics of cancer incidence a decade ago. In MCIJ2014, all of the registries prepared their data by the deadline. This means all prefectures can fix the incidences 2 years after the incident year. DCO% indicates the quality of data. If the DCO% is high, it means the cancer registry data have no detailed information about morphology, tumor stage, and treatments. In developed countries, M/I can be considered an indicator of the completeness of the data. As the latest population-based survival rate in Japan is over 60%, M/I should be around 0.40, approximately of 1-survival rate. According to the data quality in CI5 XI in the developed countries, DCO% is roughly 1% in the USA and less than 5% in European countries. The latest Japanese data are not of the highest quality, but they are comparable to them to a satisfactory extent.
By using high-quality PBCR data, rare cancer research is now restarting with several Asian countries and with the RARECARE research group in Italy, which is an international project to survey the incidence and survival of rare cancers in Europe.
Combining mortality, incidence, and survival data, the current cancer burden in the country can be determined. According to the article of Karim-Kos et al. (24), gastric cancers in both sexes and female lung cancer showed ‘artificial’ increases in incidence due to screening or improvement of PBCR data quality. Stomach cancer, colorectal cancer, and lung cancer in males may show improved treatment. Liver cancer in both sexes showed clearly lower risk factor prevalence and pre-malignant screening for hepatitis C virus (HCV), which will be described later.
Geographical disparities at these sites were associated with well-known risk factors, such as smoking, salt intake, and HCV and H. pylori infections. A high-salt diet has a synergistic effect with H. pylori infection to promote stomach cancer incidence (25,26) and results in geographical variation and a recent decrease in gastric cancer incidence (27). The differences can be partially explained by the disparity of such risk factors in Japan (28). The prevalence of antibody to HCV, for example, varied according to sex, birth year, and geographic area (29). This background explains well the high incidence of liver cancer in a specific birth cohort and in several prefectures. Adult T-cell leukemia typically occurs in the Kyushu area (30). These cancers related to infections tend to be concentrated in several areas. It is also known that socio-economic inequality leads to gaps in cancer incidence, mortality, and survival (31).
The medical system in Japan is not perfect, but at least the survival rate demonstrates a high level of care in the country. For cancer of the digestive system, Japan shows very good results. High survival in stomach cancer is considered due to the excellent technique of early detection, and the intensive diagnosis, endoscopic surgery, and high survival rates in liver cancer can be due to screening of hepatitis virus carriers, and their treatment from follow-up is also thought to be effective. The survival gap compared to European countries is thought to be due to biologic differences of the type of cancer, not a difference in the quality of the medical system.
Unfortunately, cancer screening in Japan is neither completely evidence-based nor controlled in terms of quality. It should be noted that prostate and thyroid cancers had a high proportion of detection by screening, which are not recommended by the guideline. Such “cancer screening” affects the recent increase in prostate cancer incidence. It is considered that organized screening may also be related to the high proportion of detection and the increase in incidence in thyroid cancer.
Those prefectures that have high SMR/SIR, such as Aomori prefecture, make a serious effort to identify the risk factors and control them. Their hypothesis is that low compliance of cancer patients with the results of cancer screening causes delayed diagnosis and treatment.
Need for evidence-based cancer control programs for the heavy cancer burden in Japan
Given its hyper-aging society, Japan will likely face a substantial increase in the number of elderly cancer patients. This heavy cancer burden will be compounded by the functional limitations and co-morbidities that often present in older cancer patients. In such cases, interventions suitable for younger people may not be applicable, because interventions aimed at reducing mortality will not necessarily enhance quality of life in elderly adults. The population in Japan started to decline in 2016, which was the first time a decline has been seen since the census began in 1920. This situation is very rare globally, as Japan is the only country among the top 20 most populated countries whose population is decreasing. The proportion of the population above 65 years of age will exceed 30% in 2025, whereas it is 21.2% in the other developed countries (32).
The risk of a shortage of medical resources and human resources is clear and present. The risk of a shortage of medical resources and human resources is clear and present, and the hyper-aging society and expected heavy cancer burden exacerbate the problem. A health economic analysis emphasized that breast cancer was the leading cause of increased costs due to workplace absenteeism (33). As mentioned, breast was the leading site of 5-year prevalence in females. It is necessary to establish support systems for such working cancer survivors. It is considered that one of the most important keys to overcoming the difficulty is the cancer statistics, and the data should be used effectively, not only for epidemiological research, but also for national and local cancer control planning. The MHLW has aimed for all prefectures to have a system monitoring cancer incidence. Localized cancer control activities have already been done in many prefectures. For example, as a measure against liver cancer, an HCV antibody test can be incorporated as part of the screening method for residents in hyperendemic prefectures, such as Osaka or Saga. This may result in early detection of HCV carriers in the general population, facilitating the appropriate care of such individuals before the onset of hepatitis or hepatic cancer (34). While Japan is far behind the other developed countries in tobacco control, its role as a risk factor is clear (35).
Theoretically, 100% coverage of cancer patients in the country under the NCR will contribute to rare cancer, including childhood cancer, research. For children and younger adults, cancer will be their primary concern, because they may have no other serious health problems. In such cases, it is reasonable to focus on reducing specific mortality and receiving care from cancer specialists at centralized facilities. The rare cancer center was therefore established in the NCC in 2014 to diagnose and treat rare cancer patients intensively.
The first cancer statistics from the NCR will be reported by the end of the year 2018. Some instability is expected in the first published incidence and survival rates. However, in 3 years at the latest, the cancer statistics in Japan should be stable, reliable, and complete. The estimation of cancer incidence in the MCIJ project acted as an intermediary between the prefectural cancer registry era and the national cancer registry in 2016.
Cancer statistics and cancer registration in Japan in accordance with legislation are keys to effective evidence-based cancer control. To achieve true cancer control, the use of cancer registry data should be maximized in cooperation with researchers and the private sector.
Acknowledgments
Funding: This work was supported by a Health Labour Sciences Research Grant, H29-Cancer Control-General-016.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Wanqing Chen) for the series “Global Cancer Burden” published in Annals of Cancer Epidemiology. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ace.2018.08.01). The series “Global Cancer Burden” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fujimoto I, Hanai A, Oshima A. Descriptive epidemiology of cancer in Japan: current cancer incidence and survival data. Natl Cancer Inst Monogr 1979;5-15. [PubMed]
- Matsuda T, Sobue T. Recent trends in population-based cancer registries in Japan: the Act on Promotion of Cancer Registries and drastic changes in the historical registry. Int J Clin Oncol 2015;20:11-20. [Crossref] [PubMed]
- Tanaka H, Matsuda T. Arrival of a new era in Japan with the establishment of the Cancer Registration Promotion Act. Eur J Cancer Prev 2015;24:542-3. [Crossref] [PubMed]
- Portal Site of Official Statistics of Japan website. Available online: http://www.e-stat.go.jp/
- Marugame T, Kamo K, Katanoda K, et al. Cancer incidence and incidence rates in Japan in 2000: Estimates based on data from 11 population-based cancer registries. Jpn J Clin Oncol 2006;36:668-75. [Crossref] [PubMed]
- Monitoring of Cancer Incidence in Japan 2014. Tokyo: National Cancer Center, Japan, 2018.
- Katanoda K, Shibata A, Matsuda T, et al. Childhood, adolescent and young adult cancer incidence in Japan in 2009-2011. Jpn J Clin Oncol 2017;47:762-71. [Crossref] [PubMed]
- Steliarova-Foucher E, Colombet M, Ries LAG, et al. International incidence of childhood cancer, 2001-10: a population-based registry study. Lancet Oncol 2017;18:719-31. [Crossref] [PubMed]
- Tamaki T, Dong Y, Ohno Y, et al. The burden of rare cancer in Japan: application of the RARECARE definition. Cancer Epidemiol 2014;38:490-5. [Crossref] [PubMed]
- Inoue M, Tsuji I, Wakai K, et al. Evaluation based on systematic review of epidemiological evidence among Japanese populations: tobacco smoking and total cancer risk. Jpn J Clin Oncol 2005;35:404-11. [Crossref] [PubMed]
- Inoue M, Sawada N, Matsuda T, et al. Attributable causes of cancer in Japan in 2005--systematic assessment to estimate current burden of cancer attributable to known preventable risk factors in Japan. Ann Oncol 2012;23:1362-9. [Crossref] [PubMed]
- Sasazuki S, Inoue M, Shimazu T, et al. Evidence-based cancer prevention recommendations for Japanese. Jpn J Clin Oncol 2018;48:576-86. [Crossref] [PubMed]
- Tsukuma H, Ajiki W, Ioka A, et al. Survival of cancer patients diagnosed between 1993 and 1996: a collaborative study of population-based cancer registries in Japan. Jpn J Clin Oncol 2006;36:602-7. [Crossref] [PubMed]
- Matsuda T, Ajiki W, Marugame T, et al. Population-based survival of cancer patients diagnosed between 1993 and 1999 in Japan: a chronological and international comparative study. Jpn J Clin Oncol 2011;41:40-51. [Crossref] [PubMed]
- Ito Y, Miyashiro I, Ito H, et al. Long-term survival and conditional survival of cancer patients in Japan using population-based cancer registry data. Cancer Sci 2014;105:1480-6. [Crossref] [PubMed]
- Monitoring of Cancer Incidence in Japan, survival 2006-8. Tokyo: National Cancer Center, Japan, 2016.
- Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018;391:1023-75. [Crossref] [PubMed]
- Hamashima C. Cancer screening guidelines and policy making: 15 years of experience in cancer screening guideline development in Japan. Jpn J Clin Oncol 2018;48:278-86. [Crossref] [PubMed]
- Suzuki A, Kuriyama S, Kawai M, et al. Age-specific interval breast cancers in Japan: estimation of the proper sensitivity of screening using a population-based cancer registry. Cancer Sci 2008;99:2264-7. [Crossref] [PubMed]
- Katanoda K, Hori M, Matsuda T, et al. An updated report on the trends in cancer incidence and mortality in Japan, 1958-2013. Jpn J Clin Oncol 2015;45:390-401. [Crossref] [PubMed]
- Matsuda T, Saika K. Trends of cancer incidence and mortality (Fukushima prefecture and surrounding prefectures): Graduate School of Medicine, Division of Environmental Medicine and Population Sciences, Department of Social Environmental Medicine, Osaka University, 2018. Available online: http://www2.med.osaka-u.ac.jp/envi/20180706/
- Pisani P, Bray F, Parkin DM. Estimates of the world-wide prevalence of cancer for 25 sites in the adult population. Int J Cancer 2002;97:72-81. [Crossref] [PubMed]
- Cancer Incidence in Five Continents, Vol. XI (electronic version). Bray F, Colombet M, Mery L, et al. editors. Lyon: International Agency for Research on Cancer, 2017.
- Karim-Kos HE, de Vries E, Soerjomataram I, et al. Recent trends of cancer in Europe: a combined approach of incidence, survival and mortality for 17 cancer sites since the 1990s. Eur J Cancer 2008;44:1345-89. [Crossref] [PubMed]
- Kawakubo M, Ito Y, Okimura Y, et al. Natural antibiotic function of a human gastric mucin against Helicobacter pylori infection. Science 2004;305:1003-6. [Crossref] [PubMed]
- Kato S, Tsukamoto T, Mizoshita T, et al. High salt diets dose-dependently promote gastric chemical carcinogenesis in Helicobacter pylori-infected Mongolian gerbils associated with a shift in mucin production from glandular to surface mucous cells. Int J Cancer 2006;119:1558-66. [Crossref] [PubMed]
- Ueda J, Gosho M, Inui Y, et al. Prevalence of Helicobacter pylori infection by birth year and geographic area in Japan. Helicobacter 2014;19:105-10. [Crossref] [PubMed]
- Matsuzaka M, Fukuda S, Takahashi I, et al. The decreasing burden of gastric cancer in Japan. Tohoku J Exp Med 2007;212:207-19. [Crossref] [PubMed]
- Tanaka H, Hiyama T, Tsukuma H, et al. Prevalence of second generation antibody to hepatitis C virus among voluntary blood donors in Osaka, Japan. Cancer Causes Control 1994;5:409-13. [Crossref] [PubMed]
- Nosaka K, Iwanaga M, Imaizumi Y, et al. Epidemiological and clinical features of adult T-cell leukemia-lymphoma in Japan, 2010-2011: A nationwide survey. Cancer Sci 2017;108:2478-86. [Crossref] [PubMed]
- Ito Y, Nakaya T, Nakayama T, et al. Socioeconomic inequalities in cancer survival: a population-based study of adult patients diagnosed in Osaka, Japan, during the period 1993-2004. Acta Oncol. 2014;53:1423-33. [Crossref] [PubMed]
- Department of Economic and Social Affairs PD. World Population Prospects: The 2017 Revision. New York, 2017.
- Yamauchi H, Nakagawa C, Fukuda T. Social impacts of the work loss in cancer survivors. Breast Cancer 2017;24:694-701. [Crossref] [PubMed]
- Tanaka H, Imai Y, Hiramatsu N, et al. Declining incidence of hepatocellular carcinoma in Osaka, Japan, from 1990 to 2003. Ann Intern Med 2008;148:820-6. [Crossref] [PubMed]
- Tsugawa Y, Hashimoto K, Tabuchi T, et al. What can Japan learn from tobacco control in the UK? Lancet 2017;390:933-4. [Crossref] [PubMed]
Cite this article as: Matsuda T, Saika K. Cancer burden in Japan based on the latest cancer statistics: need for evidence-based cancer control programs. Ann Cancer Epidemiol 2018;2:2.