Sociodemographic disparities in access to ovarian cancer treatment
Introduction
Ovarian cancer is the fifth most common cause of cancer death among women in the United States (1). Although advances in ovarian cancer treatment over the past several decades have led to improvements in survival, this benefit has not been realized for all populations. Among the factors that influence improved outcomes for ovarian cancer are optimal surgical treatment by a gynecologic oncologist, treatment in a high-volume hospital, and guideline-compliant treatment (2-4). Failure to receive optimal treatment and poorer survival rates have been reported for older women, African-American women, women with low income, and women with public health insurance coverage or no coverage (5-9). The role of geography in explaining disparities also has been explored. Regional differences in treatment, as well as urban-rural differences, and long travel distances, have been shown to influence the receipt of surgery or chemotherapy for ovarian cancer (4,7,10). Thus, particularly vulnerable populations experience spatial and/or sociodemographic barriers to ovarian cancer treatment.
Whereas this research demonstrates the importance of factors such as education and income as well as healthcare location and travel time in accessing optimal care, there has also been a growing interest in finding ways to integrate the spatial and non-spatial factors into a single framework. Recognizing that access to healthcare is a multidimensional construct (11,12), the aim here is to consider both spatial characteristics and socio-contextual variables in a way that provides a more comprehensive view of access to healthcare. Some advantages of a single access framework might include ease in interpreting maps or summary outcome measures and enabling the ability to identify specific geographic areas and populations with particularly high barriers to healthcare access for policy or program interventions. Challenges in creating an integrative measure include developing methods of aggregating sociodemographic variables and establishing a simple but effective process of consolidating spatial and non-spatial characteristics (13).
A number of new approaches advance our understanding of the role of geographic location in health care access since Penchansky and Thomas outlined a conceptual model of access to care (11). These have used multivariate or multilevel modeling to integrate spatial and sociodemographic characteristics based on patient-level data. An examination of colorectal cancer testing using multilevel models that included county-level sociodemographic characteristic, patient-level data, and distance to testing facilities found that patients with the longest travel distance were least likely to undergo testing (14).
Recent geographical approaches to identifying vulnerable populations have examined both a spatial accessibility component as measured by travel time or distance to services, and a sociodemographic component including factors such as income, ethnicity, race, age, and health insurance status. Spatial regression models have been used to incorporate both sets of factors to examine access to mammography facilities in Chicago, Illinois (15), and multilevel modeling used to combine demographic and community structural factors in describing regional differences in colorectal cancer screening (14). Bristow and colleagues (16) used individual level patient data, patient distance to hospitals having a high volume of ovarian cancer cases, and census block-level socioeconomic status (SES) data in generalized additive models to obtain odds of adherence to optimal treatment for ovarian cancer. Travel distance and proximity to facilities treating a high volume of ovarian cancer patients were predictive of receiving guideline-adherent treatment (16). Wang and Luo (13) conducted a study of access to primary care to demonstrate a method to integrate spatial and sociodemographic characteristics into one framework. They used a two-step floating catchment area method and factor analysis of demographic characteristics to identify and map tracts with socioeconomic disadvantage, sociocultural barriers, and high health care needs. Spatial accessibility and socioeconomic factors were combined to identify specific tracts with high physician shortages.
Building upon this current line of research, we demonstrate a method that considers spatial and sociodemographic factors separately, as well as combined geosocial vulnerability, to measure access to ovarian cancer care by a gynecologic oncologist. In our approach, we used available tract-level census data and standard GIS techniques (including geocoding, network analysis, and E2SFCA) to measure gynecologic oncologist supply and population demand in a method that is relatively easy to implement. Key inputs in this analysis are sociodemographic characteristics that can influence medical care seeking and receipt of optimal care. Thus, our approach combines spatial and non-spatial factors using a reasonably simple process (13). We then test the association between our geosocial vulnerability score using ovarian mortality rates in Georgia, US.
Methods
Measuring access to gynecologic oncologists
The enhanced two-step floating catchment area (E2SFCA), introduced by Luo and Qi (17) has been used to measure spatial accessibility that incorporates both supply and demand. This method calculates a population-to-provider ratio within a specified catchment area, in this case defined by travel time. To account for diminishing accessibility that occurs with increasing distance within a catchment area, we weighted two travel time zones within each catchment, giving a lower accessibility measure for the zone with longer travel times. Compared to other methods used to measure spatial accessibility, such as basic provider-to-population ratios or travel time impedance, researchers have found E2SFCA to be optimal for measuring healthcare access for both rural and urban populations over large geographic areas (18,19).
We used the E2SFCA to calculate the spatial accessibility of gynecologic oncologists in Georgia. First, we created catchment areas for each of Georgia’s twenty gynecologic oncologist clinics. Clinic street address locations were obtained from the Society for Gynecologic Oncology website (https:/www.sgo.org/seek-a-specialist/). One of the authors (LP) contacted offices for address verification. Addresses were geocoded using Pitney Bowes’ Centrus geocoding tool (Centrus Desktop, version 6.0). Sixty-minute street network drive time catchments from each clinic were created using NAVTEQ 2010 streets and Esri’s Network Analyst (ArcGIS 10.3). We chose a 60-minute catchment size to accommodate urban, suburban, and rural driving distances within this mid-sized state. Each 60-minute catchment was subdivided into two travel time zones, 0 to 30 minutes and greater than 30 to 60 minutes. For these two time zones, we applied a weight to account for distance decay within the 60-minute time zone. Due to the nature of accessing specialty cancer care facilities we chose a slow decay function to assign the zonal weights (17). Therefore, the 0–30-minute zone was assigned a weight of 1.0; the >30–60-minute zone, 0.8.
To calculate the population-to-provider ratio, we used the Geospatial Research Analysis and Services Program (GRASP) Population Estimator tool to estimate the number of women 15 years of age and older within each travel time zone (20). The tool applies an area proportion technique that assumes an equal population distribution within each census tract. The population of each tract is multiplied by the proportion of the tract area that falls within the travel time zone. The resulting tract population totals and proportions were summed to determine a population total for the zone. The population totals were then adjusted by the weight assigned for each travel time zone. To reach a provider-to-population ratio for each clinic, the number of physicians at each location were summed and divided by the weighted population estimates for each zone (17).
For the second step of the E2SFCA, we summed the ratios created in step one for each clinic that fell within the travel time zones from each population center. To do this, population-weighted centroids were calculated at the census tract level using 2010 Census block population data for women 15 years of age or older. Sixty-minute catchments were created for each weighted centroid, with the two travel zones of 0–30, and 30–60 minutes. The sum of the ratios was weighted for each zone, using the same zonal multipliers as in step one, giving each tract an accessibility score ranging from 0.0 (no accessibility) to 1.6 (high accessibility) per 1,000 women.
Measuring geosocial vulnerability
Wang and Luo (13) provided a methodological framework for modeling geosocial vulnerability. We based our choice of sociodemographic characteristics on prior research identifying factors associated with ovarian cancer outcomes such as income, poverty, race, and education level (4,9). Because the percentage of ovarian cancer deaths is highest among women 65 to 74 years of age (21), we included Census 2010 100% count data (SF1) percentage of residents age 65 and older (www.census.gov). In addition, we included the following American Community Survey (ACS), 2006–2010 variables: percentage of persons below the poverty level, and percentage of persons with no high school diploma for persons aged 25 and older (www.census.gov).
The indicator ‘residents age 65 and older’ was selected as the incidence of ovarian cancer increases substantially with age. Because ovarian cancer is more frequently diagnosed in white women than black women, we have excluded minority status as an informative variable (https://www.cdc.gov/cancer/ovarian/statistics/race.htm). We selected two census variables for which Georgia ranks in the bottom quintile of the nation—poverty, an indicator likely varying geographically within the state—and the absence of a high school diploma. These two variables are associated with having less social capital; that is, social networks that are less likely to provide awareness of the disease and options for seeking treatment. These three variables are positively associated with ovarian cancer mortality and statistically significant at P<0.001.
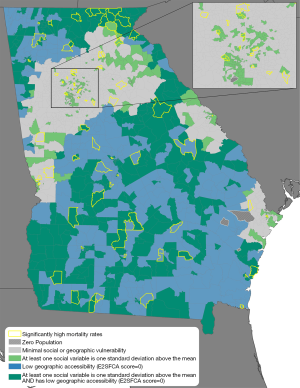
We identified the census tracts in Georgia that were one standard deviation above the mean for any of the three selected sociodemographic census variables. These tracts (N=653) were deemed socially vulnerable. The 360 tracts with low geographic accessibility (E2SFCA score of 0.0) were flagged as spatially vulnerable. Combined geosocial vulnerability indicates tracts that presented with both a geographic accessibility of 0.0 and were one standard deviation above the mean in any of the sociodemographic variables. We identified 341 tracts (17.4%) as combined geosocially vulnerable tracts.
Measure testing
Georgia ovarian cancer mortality records for Malignant Neoplasm of Ovary (ICD-10-CM C56) for the years 2005–2013 were obtained from the Georgia Department of Public Health (Georgia Department of Public Health, Georgia Ovarian Cancer Mortality Data). Mortality records had been geocoded to the street address level and then aggregated to census tract. We calculated raw ovarian cancer rates per 10,000 women for an eight-year period (2005–2013) using ovarian cancer mortality and female population 15 years of age and older by census tract. Total tract population varies considerably across the state, and such variability lends to a small numbers problem when analyzing clustering in health data. To overcome the varying degrees of reliability associated with small numbers, and because calculations with small counts can result in unstable raw mortality rates, we investigated two mapping techniques: probability mapping and spatial empirical Bayes smoothing (22).
We used the Poisson distribution method, which models the probability of rare binary events (e.g., ovarian cancer deaths), to determine if the number of deaths in a given census tract is significantly different than expected based on the state mortality rate. In other words, the Poisson test models the likelihood that the calculated tract mortality rates indicate a higher or lower probability than the expected state mortality rate. Poisson values closer to zero indicate a higher than expected mortality rate. Because probability mapping may overemphasize the significance of areas with large populations, we also explored spatial empirical Bayes estimation, which “represents a compromise between probability mapping and simple choropleth mapping of rates” (22). Bayesian smoothing adjusts mortality rates for individual tracts, depending on population size, while maintaining the overall state rate. We applied the empirical Bayes smoothing technique using a 1st order queen contiguity weighting scheme and a non-informative prior in GeoDa (23). Empirical Bayesian smoothing focuses on areas with high margins of error, moving these estimates closer to the local global average of the event rate. The raw rates (non-smoothed) have not been adjusted. Both raw and smoothed rates had relatively normal distributions.
Results
Geosocial vulnerability
Figure 1A displays the results of the E2SFCA. The map shows generally higher accessibility in urban areas, and concentrically less accessibility in suburban and rural areas. One exception is tracts in the vicinity of the sole clinic in Athens in northeastern Georgia where higher population and a single oncologist results in a lower accessibility ratio. In contrast, Gainesville, also in the northeastern part of the state, has one provider but a much lower accessibility ratio. Most of the balance of the state, consisting of rural areas and smaller cities, has an accessibility score of 0.00 (low accessibility).

Figure 1B shows an additional measure of geographic variation within the state: that of sociodemographic vulnerability. Instead of an urban-rural continuum, the results indicate a suburban effect around major population centers (e.g., Columbus, Savannah, Athens, & Augusta), with high vulnerability at the city center and less vulnerability on the periphery. In Atlanta, higher vulnerability is especially apparent in the southern part of the city. The remainder of the state, including both smaller cities and extensive rural areas, displays more variability in social vulnerability than is seen in spatial accessibility. The combined results of our measure of low geographic accessibility and high social vulnerability are represented in Figure 2, alongside higher than expected mortality rates. Low accessibility in areas remote from a clinic combined with high social vulnerability produce many tracts throughout the state that are doubly challenged with regard to access to gynecological oncologists.
Ovarian cancer mortality
Figure 3 presents maps of ovarian cancer mortality in Georgia; raw ovarian mortality rates (Figure 3A), and empirical Bayes smoothed ovarian mortality rates (Figure 3B). Additionally, both maps show areas of significantly high mortality rates calculated by the Poisson distribution test. Figure 3A shows overall spatial variability in areas of high and low raw mortality rates. Figure 3B, presenting smoothed mortality rates based on the size of the total tract population, shows less variability spatially. The raw rates and smoothed mortality rates were moderately correlated with each other (r=0.69) as well as with the Poisson probability distributions (0.929, 0.651 for raw and smoothed respectively). A paired t-test indicated no statistically significant difference between the two rates, thus providing validation for the stabilized smoothed rates. The difference in the results between the raw and smoothed rates is due to the smoothed rates being more geographically stable than the raw rates. The process yields tract rates that are closer to the local geographical average as opposed to the raw rate, which can vary considerably across tracts. Tracts identified by Poisson probability analysis as having significantly higher than expected mortality are presented alongside the geosocial vulnerability in Figure 2. These tracts are moderately distributed across the state but appear to correspond with those tracts having joint vulnerabilities.
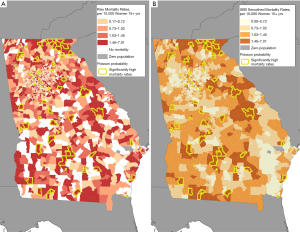
Table 1 depicts the results of the geosocial vulnerability measure for ovarian cancer mortality at the census tract level. Out of 82 tracts flagged with significantly high mortality, 75% (N=61) fell into one of the three vulnerability designations. Tracts exhibiting both high social vulnerability and low geographic accessibility, defined as geosocially vulnerable, accounted for 37% (N=30) of the high mortality tracts.
Table 1
| Vulnerability | Tracts (n) | Tracts (%) | Population (n) | Population (%) | Tracts with significantly high mortality (%) |
|---|---|---|---|---|---|
| High social vulnerability only | 312 | 16.0 | 607,817 | 12.3 | 14 (17.1) |
| Low geographic accessibility only | 360 | 18.4 | 901,369 | 18.2 | 17 (20.7) |
| Combined geosocial vulnerability | 341 | 17.4 | 681,980 | 13.8 | 30 (36.6) |
| All vulnerable tracts | 1,013 | 51.8 | 2,191,166 | 44.2 | 61 (74.4) |
| Georgia total | 1,955 | 100.0 | 4,958,477 | 100.0 | 82 (4.2) |
Discussion
Results from this analysis illustrate that highlighting both spatial and sociodemographic vulnerabilities can identify areas of healthcare access vulnerability not revealed by either spatial analysis or sociodemographic assessment alone. Whereas lower healthcare accessibility in rural areas has been well described, our maps also show considerable heterogeneity in access to care in urban areas where the disadvantaged census tracts can be easily identified. This type of analysis may be especially useful in urban areas where transportation distances are shorter but sociodemographic barriers to care limit what otherwise appears to be an adequate population to specialist ratio. Our findings are consistent with studies that have described urban disadvantages in cancer screening, diagnosis, and treatment (14,15,24-26). Importantly, we were also able to show that the 341 tracts exhibiting combined geosocial vulnerability represented 37% of tracts with high ovarian cancer mortality.
Despite a small decline in both incidence and mortality rates over the past two decades, 5-year survival remains relatively low (21,27) and without advances in screening or preventive measures, treatment with optimal surgery and chemotherapy offers best potential for long term survival (28,29). That poverty plays a role in access to care and mortality is recognized across many health outcomes. Education level may reflect knowledge about the best treatment for ovarian cancer or the need for specialist care as well as financial resources. Our findings of the association between mortality and high geosocial vulnerability suggest characteristics such as poverty and low education levels, as well as distance, impede access to that care.
We note several limitations in our study. Ours is an ecologic analysis that describes the geosocial vulnerabilities of women in a census tract with respect to medical specialist proximity. Thus, we are referring to a hypothetical patient in that tract for whom we have no individual level characteristics. Access to care is a multifactorial construct encompassing complex relationships between spatial supply of specialists and demand for services. Therefore, in addition to sociodemographic factors and geographic accessibility that we have measured, receipt of appropriate treatment can also depend on a host of individual and system level factors such as referral patterns, health literacy, patient preferences, and out-of-pocket costs, which are difficult to measure at the ecologic level. A second limitation is that although we mapped gynecologic oncology practices in neighboring states, we did not take these practices into account in our calculations of the E2SFCA. Whereas a gynecologic oncologist in a bordering state may be closer for some patients, their access to that specialist may be limited by their health insurance network coverage or higher cost for out of network care. Finally, our travel zones were based on driving distance and did not take into account barriers or benefits posed by access limited to public transportation.
Results from this study will inform ongoing research on the application of GIS methods to spatial and sociodemographic factors and add to our understanding of contextual barriers to optimal treatment. In the future, access to specialist services such as gynecologic oncologists will become increasingly important as demand for oncologic services is expected to significantly outpace supply of oncologists by 2020 (30). Thus, combined geographic and sociodemographic analyses can identify areas of particular need for healthcare services as well as provide information that may aid in the planning and evaluation of these services.
Acknowledgments
Funding: Support for this study was provided by the Division of Cancer Prevention and Control, CDC, and the Agency for Toxic Substances and Disease Registry.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ace.2019.10.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013), and was approved by the Centers for Disease Control and Prevention (CDC)’s Office of the Associate Director for Science for research determination per CDC-SA-2010-02.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- USCS Working Group. United States Cancer Statistics: 1999-2013 Incidence and Mortality Web-based Report. Atlanta GA: Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute, 2016.
- Dehaeck U, McGahan CE, Santos JL, et al. The Impact of Geographic Variations in Treatment on Outcomes in Ovarian Cancer. Int J Gynecol Cancer 2013;23:282-7. [Crossref] [PubMed]
- Goff BA, Matthews BJ, Larson EH, et al. Predictors of comprehensive surgical treatment in patients with ovarian cancer. Cancer 2007;109:2031-42. [Crossref] [PubMed]
- Mercado C, Zingmond D, Karlan BY, et al. Quality of care in advanced ovarian cancer: The importance of provider specialty. Gynecol Oncol 2010;117:18-22. [Crossref] [PubMed]
- Thrall MM, Goff BA, Symons RG, et al. Thirty-Day Mortality After Primary Cytoreductive Surgery for Advanced Ovarian Cancer in the Elderly. Obstet Gynecol 2011;118:537-47. [Crossref] [PubMed]
- Terplan M, Smith EJ, Temkin SM. Race in ovarian cancer treatment and survival: a systematic review with meta-analysis. Cancer Causes Control 2009;20:1139-50. [Crossref] [PubMed]
- Shalowitz DI, Spillman MA, Morgan MA. Interactions with industry under the Sunshine Act: an example from gynecologic oncology. Am J Obstet Gynecol 2016;214:703-7. [Crossref] [PubMed]
- Terplan M, Schluterman N, McNamara EJ, et al. Have racial disparities in ovarian cancer increased over time? An analysis of SEER data. Gynecol Oncol 2012;125:19-24. [Crossref] [PubMed]
- Bristow RE, Chang J, Ziogas A, et al. Adherence to Treatment Guidelines for Ovarian Cancer as a Measure of Quality Care. Obstet Gynecol 2013;121:1226-34. [Crossref] [PubMed]
- Polsky D, Armstrong KA, Randall TC, et al. Variation in Chemotherapy Utilization in Ovarian Cancer: The Relative Contribution of Geography. Health Serv Res 2006;41:2201-18. [Crossref] [PubMed]
- Penchansky R, Thomas JW. The concept of access: definition and relationship to consumer satisfaction. Med Care 1981;19:127-40. [Crossref] [PubMed]
- Guagliardo MF. Spatial accessibility of primary care: concepts, methods and challenges. Int J Health Geogr 2004;3:3. [Crossref] [PubMed]
- Wang F, Luo W. Assessing spatial and nonspatial factors for healthcare access: towards an integrated approach to defining health professional shortage areas. Health Place 2005;11:131-46. [Crossref] [PubMed]
- Wheeler SB, Kuo TM, Goyal RK, et al. Regional variation in colorectal cancer testing and geographic availability of care in a publicly insured population. Health Place 2014;29:114-23. [Crossref] [PubMed]
- Zenk SN, Tarlov E, Sun J. Spatial equity in facilities providing low- or no-fee screening mammography in Chicago neighborhoods. J Urban Health 2006;83:195-210. [Crossref] [PubMed]
- Bristow RE, Chang J, Ziogas A, et al. Spatial analysis of adherence to treatment guidelines for advanced-stage ovarian cancer and the impact of race and socioeconomic status. Gynecol Oncol 2014;134:60-7. [Crossref] [PubMed]
- Luo W, Qi Y. An enhanced two-step floating catchment area (E2SFCA) method. Health Place 2009;15:1100-7. [Crossref] [PubMed]
- Dai D, Wang F. Geographic disparities in accessibility to food stores in southwest Mississippi. Environment and Planning B: Planning and Design 2011;38:659-77. [Crossref]
- McGrail MR. Spatial accessibility of primary health care utilising the two-step floating catchment area method: an assessment of recent improvements. Int J Health Geogr 2012;11:50. [Crossref] [PubMed]
- Chiang A. Evaluating the performance of a filtered area weighting method in population estimate for public health studies. Thesis. Georgia State University, 2013.
- Howlader N, Krapcho M, Garshell J, et al. SEER Cancer Statistics Review, 1975-2011. National Cancer Institute, Bethesda, MD, 2014.
- Cromley EK, McLafferty S. GIS and Public Health. New York: The Guilford Press, 2012.
- Anselin L, Syabri I, Kho Y. GeoDa; an introduction to spatial data analysis. Geographical Analysis 2006;38:5-22. [Crossref]
- McLafferty S, Wang F. Rural reversal? Rural-urban disparities in late-stage cancer risk in Illinois. Cancer 2009;115:2755-64. [Crossref] [PubMed]
- Peipins LA, Graham S, Young R, et al. Time and Distance Barriers to Mammography Facilities in the Atlanta Metropolitan Area. J Community Health 2011;36:675-83. [Crossref] [PubMed]
- Peipins LA, Graham S, Young R, et al. Racial disparities in travel time to radiotherapy facilities in the Atlanta metropolitan area. Soc Sci Med 2013;89:32-8. [Crossref] [PubMed]
- Sopik V, Rosen B, Giannakeas V, et al. Why have ovarian cancer mortality rates declined? Part III. Prospects for the future. Gynecol Oncol 2015;138:757-61. [Crossref] [PubMed]
- Sopik V, Iqbal J, Rosen B, et al. Why have ovarian cancer mortality rates declined? Part II. Case-fatality. Gynecol Oncol 2015;138:750-6. [Crossref] [PubMed]
- National Academies of Science, Engineering, and Medicine (NAS) 2016. Ovarian cancers: Evolving paradigms in research and care. Washington, DC: The National Academies Press. doi:
10.17226/21841 . - Hortobagyi GNAmerican Society of Clinical Oncology. A shortage of oncologists? The American Society of Clinical Oncology Workforce Study. J Clin Oncol 2007;25:1468-9. [Crossref] [PubMed]
Cite this article as: Graham S, Hallisey E, Wilt G, Flanagan B, Rodriguez JL, Peipins L. Sociodemographic disparities in access to ovarian cancer treatment. Ann Cancer Epidemiol 2019;3:10.