
Measuring the potential impact of physical inactivity on worldwide epidemiology of colorectal and breast cancers
Introduction
According to the World Cancer Research Fund (WCRF) and the American Institute for Cancer Research (AICR) physical activity, defined as movements using skeletal muscles and needing more energy than resting, plays a substantial role in attenuating the risk of developing cancer (1). The Third Joint WCRF/AICR Expert Report recently summarized the currently available evidence on the relationship between physical activity and cancer (1), concluding that “strong” evidence exists on the association between physical inactivity and enhanced risk of colorectal or breast cancer, whilst “limited” evidence supports the association between physical inactivity and other malignancies such as esophagus, lung and liver cancers. More reliable evidence would then be needed before concluding that physical activity may influence the risk of developing many other malignant diseases (1).
The firm statements endorsed by the WCRF/AICR are supported by a large amount of reliable epidemiologic data garnered during decades of research in this field. Shaw et al. recently published the results of a large meta-analysis, encompassing 20 articles covering 18 study populations, and concluding that the relative risk (RR) of colorectal cancer is decreased by 28% [RR, 0.72; 95% confidence interval (CI), 0.39–1.32] and 44% (RR, 0.56; 95% CI, 0.39–0.80) in subjects with or without a first-degree family history of colorectal cancer, respectively (2). In another recent meta-analysis of 38 cohort studies and totaling 68,416 breast cancer cases, Chen et al. concluded that being physically active reduces by nearly 13% the risk of developing breast cancer [odds ratio (OR), 0.87; 95% CI, 0.84–0.90] (3).
One of the most effective strategies for reducing the potential impact of physical inactivity on the risk of developing (and dying for) cancer, as well as on the risk of many other human pathologies such as cardiovascular disease (4), diabetes (5) and osteoporosis (6), entails a reinforced dissemination of public health recommendations for appropriate changes in activity levels, especially in those population subgroups that are most vulnerable to physical inactivity-related risk (7). Since little is known on this last aspect to the best of our knowledge, this article aims to provide an objective assessment of the worldwide epidemiology of physical inactivity-related cancer, focused on the two types of malignancies (i.e., colorectal and breast) in which the possible benefits of physical activity are considered stronger by the WCRF/AICR.
Methods
We performed an electronic search in the Global Health Data Exchange (GHDx) registry, a large database of health-related data maintained by the Institute for Health Metrics and Evaluation (8), using the keywords “low physical activity” in the category “risk” combined with “colon and rectum cancer” and “breast cancer” in the category “cause”. The theoretical minimum-risk exposure level of physical activity has been defined by the Global Burden of Disease (GBD) study group as performing 3,000–4,500 metabolic equivalent (MET)-min/week (9). The disability-adjusted life years (DALYs) has been considered as an indicator of disease-or risk factor-attributable health loss. The database searches for physical inactivity and the two types of cancer was then combined with the epidemiologic variables “year”, “sex”, “age” and “location” (using “SDI regions”, where SDI stands for socio-demographic index). The 2017 is the last searchable year in the GHDx registry, and was hence selected for reporting updated information on physical inactivity-related cancer. The impact of physical inactivity on cancer was reported as percentage of physical inactivity-related DALYs or deaths on the total amount of DALYs or deaths caused by colorectal and breast cancers.
The output of the electronic search was downloaded in comma-separated values (CSV), imported into an Excel file (Microsoft, Redmond, WA, US) and analyzed with Analyse-it (Analyse-it Software Ltd, Leeds, UK) and MedCalc statistical software (MedCalc Software, Ostend, Belgium). Simple (Pearson’s correlation) and multiple linear regression analyses were used for assessing potential associations (and their relative 95% CI) among different epidemiologic measures, whilst the risk was expressed as OR and relative 95% CI. The study was performed in accordance with the Declaration of Helsinki and under the terms of relevant local legislation. Ethics board approval is unnecessary at the local institution (University Hospital of Verona) for articles based on free scientific databases searches.
Results
According to the last searchable GDHx period (i.e., year 2017), the contribution of physical inactivity on the current burden of disability-adjusted life year (DALYs) and mortality is currently estimated at 3.44% and 3.64% for colorectal cancer, and at 1.43% and 1.54% for breast cancer, respectively. Regarding the sex distribution of physical inactivity-related colorectal cancer, the impact on DALYs (3.54% versus 3.35%; OR, 1.06 and 95% CI, 1.05–1.07; P<0.001) and deaths (3.77% versus 3.54%; OR, 1.07; 95% CI, 1.04–1.09; P<0.001) was found to be slightly more prevalent in women than in men. The age-dependent effect of physical inactivity-related DALYs and deaths caused by colorectal and breast cancers is summarized in Figure 1. The effect of physical inactivity-related on DALYs and deaths caused by the two cancers remains substantially stable, around 2.9% for colorectal cancer and 1.2% for breast cancer, up to 50 years of age, but then displays a virtually linear increase after that age (r=0.98 and 95% CI, 0.88–1.00 for colorectal cancer; r=0.98 and 95% CI, 0.90–1.00 for breast cancer; both P<0.001). A substantial relationship can be observed between the contribution of physical inactivity to the epidemiologic burden of colorectal and breast cancers, whereby the impact of sedentary life on the risk of these malignancies grows in parallel with the SDI (Table 1). Countries with the highest level of SDI are those where the impact of physical inactivity on the risk of colorectal and breast cancers is considerably higher.
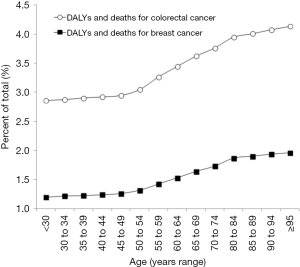
Table 1
| SDI | Colorectal cancer | Breast cancer | |||||||
|---|---|---|---|---|---|---|---|---|---|
| DALYs (%) | OR | Deaths (%) | OR | DALYs (%) | OR | Deaths (%) | OR | ||
| Global | 3.44 | – | 3.64 | – | 1.43 | – | 1.54 | – | |
| Low | 3.16 | – | 3.39 | – | 1.23 | – | 1.31 | – | |
| Low to middle | 3.21 | 1.01 (95% CI, 1.00–1.03; P=0.022) | 3.43 | 1.01 (95% CI, 0.95–1.08; P=0.706) | 1.27 | 1.03 (95% CI, 1.02–1.05; P<0.001) | 1.36 | 1.04 (95% CI, 0.95–1.14; P=0.393) | |
| Middle | 3.23 | 1.02 (95% CI, 1.01–1.03; P=0.001) | 3.45 | 1.02 (95% CI, 0.96–1.07; P=0.542) | 1.29 | 1.05 (95% CI, 1.03–1.07; P<0.001) | 1.39 | 1.06 (95% CI, 0.97–1.16; P=0.167) | |
| Middle to high | 3.62 | 1.14 (95% CI, 1.13–1.16; P<0.001) | 3.78 | 1.12 (95% CI, 1.06–1.18; P<0.001) | 1.53 | 1.24 (95% CI, 1.22–1.26; P<0.001) | 1.61 | 1.24 (95% CI, 1.13–1.35; P<0.001) | |
| High | 3.95 | 1.25; (95% CI, 1.24–1.26; P<0.001) | 4.11 | 1.21 (95% CI, 1.15–1.28; P<0.001) | 1.79 | 1.46 (95% CI, 1.44–1.48; P<0.001) | 1.88 | 1.44 (95% CI, 1.33–1.56; P<0.001) | |
SDI, socio-demographic index; DALYs, disability-adjusted life years; OR, odds ratio; CI, confidence interval.
In univariate analysis, physical inactivity-related DALYs and deaths caused by colorectal cancer were significantly associated with female sex (r=0.20; 95% CI, 0.04–0.35; P=0.017), advanced age (r=0.82; 95% CI, 0.76–0.87; P<0.001) and higher SDI (r=0.38; 95% CI, 0.23–0.52; P<0.001). These associations remained statistically significant also in multiple linear regression analysis (all P<0.01). Physical inactivity-related DALYs and deaths caused by breast cancer were then significantly associated with advanced age (r=0.80; 95% CI, 0.70–0.87; P<0.001) and higher SDI (r=0.51; 95% CI, 0.32–0.66). Also, these associations remained statistically significant in multiple linear regression analysis (all P<0.01). The cumulative effect of age, sex and SDI explained 93% of all physical inactivity-related DALYs and deaths caused by colorectal cancer, whilst the combination of age and SDI explained 94% of all physical inactivity-related DALYs and deaths caused by breast cancer.
Discussion
Colorectal and breast cancers are severe and highly prevalent human pathologies, affecting millions of subjects worldwide (10). The most recent statistic of the World Health Organization (WHO) attests that breast (2.08 million cases) and colorectal (1.80 million cases) malignancies are the second and the third most frequent forms of cancer around the world, preceded only by lung cancer (2.09 million cases) (11). Altogether these two cancers account for 1.49 million worldwide deaths each year. Notably, in its recent report the WHO also emphasizes that nearly one-third of all cancer deaths may be attributable to five major risk factors, i.e., high body mass index, low consumption of fruits and vegetables, tobacco, alcohol and—last but not least—physical inactivity (11).
The potential association between low levels of physical activity and the risk of developing cancer is ancient. In 1945, for example, Harold Morris postulated that “ample” physical exercise may be an effective strategy for fostering cancer prevention (12). Since then, an enormous volume of evidence-based information has been garnered for supporting the concept that muscular exercise produces a kaleidoscope of beneficial effects on several biological pathways, targeting specific endocrinologic, immunologic and metabolic processes which actively interplay with the pathogenesis of several forms of cancer. Being more physically active and reducing the amount of sedentary time has hence become a healthcare mantra, for expectations to lower the worldwide burden of malignant diseases (1). Although evidence is strongly emerging that the favorable effects of physical activity may not be certainly limited to colorectal and breast cancers (13), the joint WCRF/AICR Expert Report has recently recognized that the overall evidenced for other malignancies is limited, either in volume or for methodological flaws (1). These conclusions have then been endorsed by the WHO (14), the European Union (15), the US National Institute of Cancer (16) and the HK-based WCRF (17). Therefore, dissemination of public health recommendations for appropriate changes in activity levels becomes crucial, especially in population subgroups more vulnerable to physical inactivity-related cancer risk.
Taken together, the results of our analysis on the GDHx registry would contribute to define a clear picture of the physical inactivity-related risk of colorectal and breast cancers, which appears to grow in parallel with age and SDI, and seems also higher in women concerning colorectal cancer.
Genetics is one of the most reasonable explanations for the age-dependent impact of physical inactivity on colorectal and breast cancer risk. Both these cancers include some relatively frequent hereditary forms, which are typically characterized by early onset, usually before 45–50 years of age (18,19). Such a strong impact of genetic abnormalities and pathogenic mutations on the risk of developing these two malignancies would ultimately render the contribution of physical inactivity less relevant before the middle age. Another potential mechanism calls for a gradual decline of the physically active lifestyle that accompanies ageing (20), so that the impact of an inactive lifestyle on the overall risk of health loss, including developing cancer and dying for it, becomes progressively higher in the elderly.
A second important aspect that has emerged from our analysis is that women are more likely to develop physical inactivity-related colorectal cancer than men. This can be perhaps explained with the worldwide evidence that women less frequently met the currently recommended physical activity thresholds (21,22), so that the impact of physical inactivity on the risk of developing this type of cancer may be ultimately amplified in the female sex.
The relationship between physical inactivity-related cancer risk and SDI deserves special scrutiny. Notably, we found that the risk of physical inactivity-related DALYs and deaths was over 1.20-fold higher for colorectal cancer and over 1.40-fold higher for breast cancer when subjects living in high SDI countries were compared with those residents in low SDI countries (Table 1). This would actually imply that the impact of physical inactivity on the risk of developing cancer is magnified in countries with higher socioeconomic status. A probable explanation can be that the burden of insufficient physical activity is more than double in high-income than in low-income countries (21), whilst the recommended levels of physical activity is more frequently met in middle-to-low SDI countries (23). This would finally lead us to conclude that people living in middle-high and high SDI countries exercise less and, therefore, would be at enhanced risk of developing physical inactivity-related colorectal and breast cancers.
In conclusion, our analysis shows that the contribution of physical inactivity to the current burden of colorectal and breast cancer is seemingly higher in the elderly, in people living in middle-to-high SDI countries, and in the female sex for colorectal cancer.
Acknowledgments
Fabian Sanchis-Gomar is supported by a postdoctoral contract granted by “Subprograma Atracció de Talent- Contractes Postdoctorals de la Universitat de València.”
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ace.2019.09.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Physical activity and the risk of cancer. Accessed: August 6, 2019. Available online: https://www.wcrf.org/dietandcancer
- Shaw E, Farris MS, Stone CR, et al. Effects of physical activity on colorectal cancer risk among family history and body mass index subgroups: a systematic review and meta-analysis. BMC Cancer 2018;18:71. [Crossref] [PubMed]
- Chen X, Wang Q, Zhang Y, et al. Physical Activity and Risk of Breast Cancer: A Meta-Analysis of 38 Cohort Studies in 45 Study Reports. Value Health 2019;22:104-28. [Crossref] [PubMed]
- Li J, Siegrist J. Physical activity and risk of cardiovascular disease--a meta-analysis of prospective cohort studies. Int J Environ Res Public Health 2012;9:391-407. [Crossref] [PubMed]
- Hamasaki H. Daily physical activity and type 2 diabetes: A review. World J Diabetes 2016;7:243-51. [Crossref] [PubMed]
- Moayyeri A. The association between physical activity and osteoporotic fractures: a review of the evidence and implications for future research. Ann Epidemiol 2008;18:827-35. [Crossref] [PubMed]
- Clague J, Bernstein L. Physical activity and cancer. Curr Oncol Rep 2012;14:550-8. [Crossref] [PubMed]
- Institute for Health Metrics and Evaluation. Global Health Data Exchange. Accessed: August 7, 2019. Available online: http://ghdx.healthdata.org/gbd-results-tool
- GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1923-94. [Crossref] [PubMed]
- Torre LA, Siegel RL, Ward EM, et al. Global Cancer Incidence and Mortality Rates and Trends--An Update. Cancer Epidemiol Biomarkers Prev 2016;25:16-27. [Crossref] [PubMed]
- World Health Organization. Cancer - Key facts. Accessed: August 7, 2019. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer
- Morris HP. Ample exercise and a minimum of food as measures for cancer prevention? Science 1945;101:457-9. [Crossref] [PubMed]
- Moore SC, Lee IM, Weiderpass E, et al. Association of Leisure-Time Physical Activity With Risk of 26 Types of Cancer in 1.44 Million Adults. JAMA Intern Med 2016;176:816-25. [Crossref] [PubMed]
- World Health Organization. Global recommendations on physical activity for health. Accessed: August 7, 2019. Available online: https://www.who.int/dietphysicalactivity/factsheet_recommendations/en/
- European Union. Physical activity and sedentary behaviour. Accessed: August 7, 2019. Available online: https://ec.europa.eu/jrc/en/health-knowledge-gateway/promotion-prevention/physical-activity
- National Institute of Cancer. Physical Activity and Cancer. Accessed: August 7, 2019. Available online: https://www.cancer.gov/about-cancer/causes-prevention/risk/obesity/physical-activity-fact-sheet
- World Cancer Research Fund. A blueprint to beat cancer. Accessed: August 7, 2019. Available online: https://www.wcrf-hk.org/hk-en/blueprint-beat-cancer
- Lynch HT, Shaw TG. Practical genetics of colorectal cancer. Chin Clin Oncol 2013;2:12. [PubMed]
- Apostolou P, Fostira F. Hereditary breast cancer: the era of new susceptibility genes. Biomed Res Int 2013;2013:747318 [Crossref] [PubMed]
- Langhammer B, Bergland A, Rydwik E. The Importance of Physical Activity Exercise among Older People. Biomed Res Int 2018;2018:7856823 [Crossref] [PubMed]
- Guthold R, Stevens GA, Riley LM, et al. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1·9 million participants. Lancet Glob Health 2018;6:e1077-86. [Crossref] [PubMed]
- Sun F, Norman IJ, While AE. Physical activity in older people: a systematic review. BMC Public Health 2013;13:449. [Crossref] [PubMed]
- Stalsberg R, Pedersen AV. Are Differences in Physical Activity across Socioeconomic Groups Associated with Choice of Physical Activity Variables to Report? Int J Environ Res Public Health 2018; [Crossref] [PubMed]
Cite this article as: Mattiuzzi C, Sanchis-Gomar F, Lippi G. Measuring the potential impact of physical inactivity on worldwide epidemiology of colorectal and breast cancers. Ann Cancer Epidemiol 2019;3:9.


