Cancer burden in the United States—a review
Introduction
Cancer is a major public health problem in the United States. It is the second leading cause of death—with 595,930 deaths occurring in 2015 alone—following heart disease (1). Herein, we review the current cancer burden and recent trends in cancer incidence, mortality, and survival in the United States. We also provide information on factors contributing to trends in cancer occurrence and disparities, including prevalence and trends in major potentially modifiable risk factors for cancer, uptake of recommended cancer screening tests, access to treatment, and health insurance coverage.
Cancer occurrence
Population-based cancer occurrence data were obtained from a review of previously published annual reports by the American Cancer Society, the National Cancer Institute (NCI), the North American Association of Central Cancer Registries (NAACCR), and the Centers for Disease Control and Prevention (CDC) (1-4). These reports compile contemporary cancer incidence rates and trends based on data from the CDC’s National Program of Cancer Registries (NPCR) and/or the NCI’s Surveillance, Epidemiology and End Results (SEER) Registries, which combined provide complete cancer registration coverage for the United States. Select reports also provide mortality data based on death certificate information compiled by the CDC’s National Center for Health Statistics’ (NCHS) National Vital Statistics System (1,2,4). Cancer survival data were based on the Cancer in North America Survival data, compiled by NAACCR using combined data from SEER and NPCR registries (5). In the United States, projected complete cancer prevalence estimates are produced every three years by the NCI and the American Cancer Society and are currently available through 2016 (6). Importantly, the statistics presented herein do not include non-melanoma skin cancer cases, which are not required to be reported to population-based cancer registries. However, an estimated 3 million people were treated for non-melanoma skin cancer (keratinocyte carcinomas) in 2012, making it the most commonly diagnosed cancer in the United States (7). Statistics herein also do not include benign or in situ cancer of any type except for urinary bladder.
In this section, we provide information on overall cancer incidence, mortality, survival, prevalence, and disparities, followed by a brief description of childhood cancers (ages <15 years).
Cancer incidence
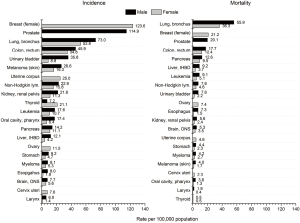
In the United States, about one in three persons are expected to be diagnosed with cancer in their lifetime, and more than 1.6 million new invasive cases of cancer (excluding non-melanoma skin cancers) are diagnosed annually (1). During the most recent 5 years of available data (2010–2014), the average age-standardized cancer incidence rate per 100,000 in the United States was about 20% higher in men compared to women (501.9 versus 417.9) (1). In general, rates for cancer types that occur in both sexes are higher in men than in women with some exceptions (e.g., breast, thyroid) (Figure 1).
Aside from non-melanoma skin cancer, the most commonly diagnosed cancers in the United States are prostate cancer in men and breast cancer in women, followed by cancers of the lung and bronchus (lung) and colorectum in either sex (1,3). During the most recent 10 years of available data (2005–2014), overall age-standardized cancer incidence rates declined by about 2% annually in men and were stable in women (1), largely reflecting trends for the most common cancers. Among men, overall declines are driven by decreasing prostate, lung, and colorectal cancer rates, whereas among women, declines in lung and colorectal cancer rates are offset by increasing rates of breast, thyroid, and corpus uteri cancers. Annual declines in lung cancer rates from 2005–2014 were slightly larger among men (2.5%) than women (1.2%), largely reflecting a greater decrease in male smoking prevalence in the past few decades (1). The precipitous decline in prostate cancer incidence rates in recent years (about 10% annually from 2010–2014) is likely due to a reduction in prostate cancer screening use resulting from recent recommendations against routine use of the prostate specific antigen test (8), discussed in further detail below.
In contrast to declining trends for most cancer types, age-standardized incidence rates increased during 2005–2014 for cancers of the female breast (0.4% annually), liver and intrahepatic bile duct (about 3% in both sexes), pancreas (about 1% in both sexes), thyroid (about 4% in both sexes), and corpus uteri (1.2%) and for melanoma of the skin among men (1.8%) (1). Incidence rates appear to also be increasing for cancer of the oral cavity and pharynx, leukemia, and myeloma in both sexes (2).
For some cancer types, incidence trends vary strikingly by subtype or age group. For example, while the incidence rate of non-cardia gastric cancer is declining in the United States, the rate for gastric cardia cancer increased during 2007–2014 (9). Further, despite decreasing incidence trends for non-cardia gastric cancer, rates are increasing among younger women (9). As another example, overall declines in colorectal cancer incidence rates mask rising rates among whites aged 20–54 years, which are increasing for reasons not well understood but possibly related to the obesity epidemic in the United States (10), as discussed further below.
Cancer mortality
Similar to incidence, age-standardized cancer death rates per 100,000 during 2011–2015 were higher in men compared to women (196.7 versus 139.5, respectively) (1). Cancer death rates are highest for lung in either sex, followed by prostate and colorectal cancers among men and breast and colorectal cancers among women (Figure 1) (4).
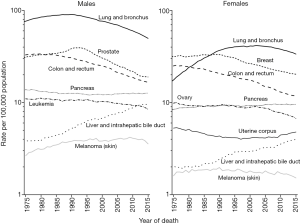
After increasing throughout much of the 20th century as a result of the tobacco epidemic, the overall age-standardized cancer death rate in the United States declined 26% from 1991 to 2015 (from 215.1 to 158.6 per 100,000) (1). This decline has been largely attributed to reductions in tobacco use and improvements in prevention, early detection, and treatment (1,2). During the most recent 10 years with available data (2006–2015), the overall cancer death rate declined by about 1.5% per year (1.8% in men vs. 1.4% in women) (1). Similar to recent incidence trends, death rates continued to decline for most cancers, including cancers of the colorectum (annual change of about 2.5% in both sexes), lung (3.3% in men and 1.9% in women), and prostate (2.9%), and increased for cancers of the liver and intrahepatic bile duct (2.3% in both sexes) and corpus uteri (1.6%) (Figure 2) (1). Death rates for melanoma of the skin declined among women only (1.8% per year), whereas pancreatic cancer death rate increased among men only (0.3%) (1). Despite a slight increase in female breast cancer incidence rates in recent years, death rates continued to decline during 2006–2015 (1.8% annually) (1).
Cancer survival
Five-year relative survival rates for all cancers combined and for many cancer types have been increasing in the United States for several decades (1,2). Among cancer patients diagnosed during 2007–2013, 5-year age-standardized relative survival was particularly high for cancers of the prostate (97%), melanoma of the skin (90%), and female breast (89%) (5), in part reflecting the large proportion of these cancer types diagnosed at a localized stage. It should be noted that despite a high 5-year survival rate, prostate cancer is among the three leading causes of cancer death among men in the United States. One reason for this difference could be the very high incidence rate of prostate cancer in the United States (114.9 per 100,000 men). Although the majority of prostate cancer cases are diagnosed at an early stage, the high mortality rate for the disease (20.1 per 100,000 in 2010–2014) (11) may in part be explained the substantial burden of distant stage disease, for which 5-year survival is relatively low. It should be noted that a rapid increase in 5-year relative survival for prostate cancer from 83% in the late 1980s to 93% in the early 1990s and to 99% in the 2000s largely reflects lead time and over-diagnosis (12). However, a recent decline in the use of prostate-specific antigen testing (12) following changes in screening recommendations in the United States (discussed below) might affect prostate cancer survival rates by a possible reduction in detection of preclinical disease.
One the other hand, 5-year age-standardized relative survival rates remain low for other relatively common cancer types, such as pancreas (11%), liver (17%), esophagus (19%), and lung (21%) (5). Many of these cancer types are not only frequently diagnosed at an advanced stage, for which survival remains poor, but also continue to have comparatively low survival for early stage disease despite treatment advances. For example, the overall 5-year relative survival rate for liver cancer more than doubled from the early 1990’s to the most recent time period but remains low (2), with the increase largely confined to localized (from 17% to 39%) and regional (from 8% to 14%) stage disease and little improvement for distant stage disease (13).
Cancer prevalence
As of January 1, 2016, more than 15.5 million persons were living in the United States with a history of cancer, a number that is expected to increase to at least 26.1 million persons by 2040 due to the growth and aging of the United States population alone (14). The most prevalent cancers among men in 2016 were prostate (3.31 million), colorectum (0.72 million) and melanoma of the skin (0.61 million). Among women, the most prevalent cancers were breast (3.56 million), uterine corpus (0.76 million) and colorectum (0.72 million). Despite lower incidence rates in women compared to men, cancer prevalence is higher among women (8.16 million) than among men (7.38 million) in the United States largely due to differences in cancer type distribution and life expectancy.
Disparities in cancer occurrence
The estimated population of the United States as of July 1, 2017 was about 325.7 million; the proportion of major racial/ethnic groups was 62% non-Hispanic (NH) white, 18% Hispanic, 13% NH black, 6% Asian/Pacific Islander, and 1% American Indian/Alaska Native (AI/AN) (15). The median household income in 2016 was $57,617 in the United States, but with a wide variation across racial/ethnic groups and states; it was $80,720 among Asians, $63,155 among NH whites, $46,882 among Hispanics, and $38,555 among blacks nationally, while by state it ranged from $78,945 in Maryland to $41,754 in Mississippi (16).
Among the major racial/ethnic groups in the United States, incidence and death rates among men overall are highest in NH blacks but lowest in Asians/Pacific Islanders (Table 1) (1,4). Among women, cancer incidence rates are highest in NH whites, whereas death rates are highest in NH blacks. While Asians/Pacific Islanders and Hispanics have lower cancer incidence and death rates overall, they have higher rates of infection-related cancers (e.g., stomach, liver), reflecting rates in immigrant countries of origin. AIs/ANs notably have the highest kidney cancer rates in the United States, potentially due to the prevalence of risk factors such as obesity and cigarette smoking (17). However, aggregated AI/AN rates mask wide variation by subregion. For example, colorectal cancer incidence rates in Alaska Natives are more than double those of NH whites and are 80% higher than those in NH blacks, whereas rates in other American Indian groups combined are similar to NH whites (18). Substantial heterogeneity in cancer rates has also been noted among Hispanic and Asian/Pacific Islander subgroups (19,20).
Table 1
| Cancer by sex | All race/ethnicities | NH white | Black | Asian/PI | Hispanic | AI/AN |
|---|---|---|---|---|---|---|
| Male | ||||||
| All cancers | ||||||
| Incidence | 501.9 | 510.7 | 560.9 | 302.8 | 386.3 | 425.3 |
| Mortality | 200.5 | 204.1 | 247.3 | 122.7 | 142.6 | 184.0 |
| Lung and bronchus | ||||||
| Incidence | 73.0 | 75.9 | 87.9 | 45.2 | 40.6 | 71.9 |
| Mortality | 55.9 | 58.4 | 68.0 | 31.7 | 27.3 | 46.3 |
| Prostate | ||||||
| Incidence | 114.9 | 107.0 | 186.8 | 58.4 | 97.0 | 78.3 |
| Mortality | 20.1 | 18.7 | 42.0 | 8.8 | 16.5 | 19.4 |
| Colon and rectum | ||||||
| Incidence | 45.9 | 45.2 | 56.4 | 37.0 | 41.9 | 50.1 |
| Mortality | 17.7 | 17.3 | 25.3 | 12.4 | 15.0 | 19.5 |
| Liver and intrahepatic bile duct | ||||||
| Incidence | 12.1 | 10.0 | 17.2 | 20.0 | 19.8 | 20.1 |
| Mortality | 9.2 | 8.0 | 13.0 | 14.3 | 13.1 | 14.9 |
| Female | ||||||
| All cancers | ||||||
| Incidence | 417.9 | 436.0 | 407.4 | 287.6 | 329.6 | 388.7 |
| Mortality | 141.5 | 145.5 | 161.8 | 88.8 | 97.7 | 129.3 |
| Lung and bronchus | ||||||
| Incidence | 52.8 | 57.6 | 50.1 | 27.9 | 25.2 | 55.9 |
| Mortality | 36.3 | 39.9 | 34.6 | 18.0 | 13.4 | 30.8 |
| Breast | ||||||
| Incidence | 123.6 | 128.7 | 125.5 | 90.8 | 91.9 | 100.7 |
| Mortality | 21.2 | 21.2 | 29.2 | 11.3 | 14.4 | 14.1 |
| Colon and rectum | ||||||
| Incidence | 34.8 | 34.5 | 41.7 | 27.0 | 29.3 | 41.3 |
| Mortality | 12.4 | 12.3 | 16.5 | 8.8 | 9.2 | 14.0 |
| Liver and intrahepatic bile duct | ||||||
| Incidence | 4.2 | 3.4 | 5.1 | 7.6 | 7.6 | 8.8 |
| Mortality | 3.7 | 3.3 | 4.5 | 6.1 | 5.8 | 6.9 |
Racial/ethnic minorities also experience disparities in cancer survival. The 5-year relative survival rate for all cancers combined in 2007–2013 was 68% in whites versus 61% in blacks, with striking differences for specific cancer types, such as uterine corpus (84% vs. 62%, respectively) (1). Although blacks are more likely than whites to be diagnosed with cancer at advanced stages, stage-specific survival rates for most cancers are also lower in blacks, reflecting in part further disparities in access to and receipt of high quality treatment (1). Disparities by socioeconomic status and race/ethnicity are particularly evident in ages <65 years, who are typically not covered by Medicare health insurance and may experience more barriers to high quality care (1,21). In some instances, however, racial/ethnic disparities in cancer occurrence in the United States persist even after adjusting for socioeconomic differences. In one study, contemporary colorectal cancer death rates in NH blacks were higher than those in NH whites even when educational attainment, a proxy measure for socioeconomic status, was similar (22). Factors contributing to racial/ethnic disparities in cancer occurrence after adjustments for socioeconomic status may include racial/ethnic differences in susceptibility and tumor biology; the use and availability of preventive care, screening, and treatment; and quality of cancer care received even within the same socioeconomic level (23-25).
As an example of the substantial geographic variation in cancer disparities, in 2015, cancer death rates in ages <65 years in Massachusetts were lower in NH blacks than in NH whites, whereas rates in the District of Columbia were nearly three-times higher in NH blacks (1). Some cancer disparities occur in cross-state regions. For example, county-level analyses have demonstrated that cancer death rates in certain regions in the United States, such as parts of Appalachia, have lagged behind national declines for lung (among women) and colorectal cancers (26,27).
Childhood cancer
Cancer in children <15 years is rare in the United States, accounting for less than 1% of all newly diagnosed cancer cases and 2% of cancer deaths estimated to occur in the United States in 2018 (1). The average age-standardized cancer incidence rate per 100,000 in children aged <15 years during 2009–2013 was 16.5, ranging from 11.5 in AIs/ANs to 17.1 in NH whites (2). During that period, incidence rates increased in all race/ethnicities combined by 0.8% per year on average, with the annual percent increases ranging from 0.4% among Hispanics to 1.5% among blacks; rates remained stable in AIs/ANs (2). Among all cancers in ages <15 years (including benign and borderline malignant brain tumors), leukemia is the most common malignancy, accounting for 29% of cancers, followed by brain and other nervous system tumors (26%) and lymphomas and reticuloendothelial neoplasms (12%); approximately one-half of the latter group are non-Hodgkin lymphomas (including Burkitt lymphoma) and one-quarter are Hodgkin lymphomas (1).
Cancer is the second leading cause of death in children ages 1 to 14 years in the United States, following accidents (1). Average cancer death rates in ages <15 years during 2010–2014 was 2.1 per 100,000, ranging from 1.8 in Asians/Pacific Islanders and AIs/ANs to 2.2 in whites (2). In contrast to recent incidence rates, cancer death rates during 2010–2014 declined in all racial/ethnic groups except AIs/ANs, for which the average annual percent change was not calculated due to sparse data. The average decline per year in all race/ethnicities combined was 1.6%, ranging from 1.5% in whites to 2.6% in Asians/Pacific Islanders (2). The 5-year relative survival rate during 2007–2013 for all cancers combined in ages <15 years was 83%; the rate was >90% for lymphoid leukemia (91%), non-Hodgkin (91%) and Hodgkin (98%) lymphoma, and renal tumors (92%), but was lower for acute myeloid leukemia (65%), central nervous system neoplasms (73%), soft tissue sarcomas (75%), and neuroblastoma (79%) (1).
Factors contributing to trends in cancer occurrence and disparities
Trends and differences in exposure to cancer risk factors and access to and utilization of cancer screening and treatment can largely explain trends in cancer incidence, death, and survival rates and variations by race/ethnicity, geographic location, and socioeconomic status in the United States. Exposure to risk factors is the major driver of trends and inequalities in cancer incidence, except for cervical and colorectal cancer, for which cancer screening have greatly contributed to a decline in incidence rates by detecting precancerous lesions (28). Increased screening for prostate cancer, however, was the main contributor to a substantial increase in prostate cancer incidence rates in the 1990s (29). Trends and inequalities in cancer death rates in many cases are also largely driven by patterns of exposure to risk factors, particularly for cancers with little improvement in survival. An example is the substantial decrease in lung cancer death rates following declines in smoking prevalence in the United States, as well as differences in lung cancer mortality by sex, race/ethnicity, and geography according to smoking prevalence in corresponding subpopulations (30). For cancers with a great improvement in survival, cancer screening (e.g., cervical cancer) and/or improvement in treatment (e.g., childhood hematologic malignancies) generally play an important role in declining mortality rates (1,28). A combination of these factors may determine trends and inequalities in cancer occurrence for some other cancers.
In this section, we review trends in prevalence of potentially modifiable risk factors of cancer and some of the recommended measures to reduce the exposure in the United States. We also briefly discuss the status of cancer screening and treatment. Finally, we provide information on health insurance coverage as one of the main indicators of access to cancer preventive care, screening, and treatment in the United States.
Potentially modifiable risk factors of cancer
A substantial proportion of cancers in the United States are caused by potentially modifiable risk factors and thus could be prevented (31-34). This proportion was estimated to be about 42% of all incident cancers (excluding non-melanoma skin cancers) and 45% of all cancer deaths in 2014; the corresponding proportions for cases and deaths by sex were 43% and 48% among men, respectively, and 42% among women (34).
The proportion of specific cancer types attributable to potentially modifiable risk factors varies greatly. For example, the proportion of attributable cancer cases in both sexes combined is more than 80% for cervical cancer, Kaposi sarcoma, melanoma of the skin, and cancer of the anus, lung and bronchus, and larynx but 5% or less for Hodgkin’s lymphoma and ovarian cancer (34). For some cancers, such as brain cancer, few major modifiable risk factors have been established.
Smoking
Smoking is the greatest contributor to cancer incidence and mortality in the United States despite a substantial decline in prevalence during past decades (34-36). An estimated 19% of all cancer cases (24% in men and 15% in women) and 29% of all cancer deaths (33% among men and 24% among women) are attributable to cigarette smoking (34). However, this proportion varies across states. Among men, the proportion of cancer deaths attributable to smoking is about 30% or more in all states (except Utah, 22%), but it is greater in southern states (38–40% in Kentucky, West Virginia, Tennessee, Louisiana, and Arkansas) (36). Among women, the proportion ranges from 11% in Utah and 19% in Hawaii and California to 26–29% in Tennessee, Arkansas, Nevada, Alaska, and Kentucky (36).
There is also a substantial disparity in smoking prevalence and related cancer burden by socioeconomic status in the United States (30,37). For example, age-adjusted prevalence of current cigarette smoking among NH white men aged ≥25 years in 2015 ranged from 7% among those with a Bachelor’s or higher to 27% among those with no high school diploma (38). Likewise, average annual age-adjusted death rates per 100,000 in 2008–2010 for lung cancer in NH white men ranged from 8.8 among those with ≥16 years of education to 52.8 among those with ≤12 years of education (30). Similar disparities in smoking prevalence and related cancer burden exist across states and racial/ethnic groups (30,38). There is also wide variation across states in the number and intensity of implemented tobacco control measures, including excise taxes, which appear to have the strongest effect in the United States, followed by smoke-free laws (39,40). Some proven strategies to reduce smoking include tobacco taxation, promotion of tobacco-free environments and smoking cessation, implementation of warning labels (notably with color graphics) and mass-media education campaigns, and tobacco advertisement bans (41). No state has yet fully implemented those measures at the levels recommended by CDC (39).
Excess body weight, alcohol, poor diet, and physical inactivity
Excess body weight accounts for the second highest proportion of all cancer cases and deaths in the United States, preceded by smoking, about 8% of cancer cases (5% in men and 11% in women) and 7% of cancer deaths (6% in men and 7% in women) (34). From the 1960s to 2013–2014, the prevalence of obesity (BMI ≥30 kg/m2) and class 3 obesity (BMI ≥40 kg/m2) in the United States increased from 13% to 38% and from 1% to 8%, respectively, while overweight (BMI 25.0–29.9 kg/m2) prevalence remained high at 32–34% (42,43). By race/ethnicity, obesity prevalence among men is similar in NH whites, blacks, and Hispanics (35%-38%), but is lower in Asians (12%) (43). Among women, obesity prevalence ranges from 13% in Asians to 38% in NH whites, 47% in Hispanics, and 57% in blacks (43). Among college graduates, obesity prevalence is lower among NH white men and women, NH black women, and Hispanic women, whereas there is no statistically significant difference in obesity prevalence by education level among NH black and Hispanic men and Asian men and women (44). By state, obesity prevalence in 2014 ranged from 21% in Colorado to 36% in Arkansas (45).
Nearly 6% of all cancer cases (5% in men and 6% in women) and 4% of all cancer deaths in both sexes are attributable to alcohol drinking (34). Alcohol-related cancer is mainly associated with excessive drinking, which includes binge drinking (≥4 and ≥5 drinks per occasion for a woman or a man, respectively) and heavy drinking (≥8 and ≥15 drinks per week for a woman or a man, respectively) (46). Approximately 38 million people (17% of adults) binge drink in the United States, making it the most common type of excessive alcohol drinking in the country (46). Past-month binge drinking prevalence among adults aged ≥50 years saw a relative increase of 19% from 2005–2006 to 2013–2014 (47). Although binge drinking is more common among men, the increase in prevalence occurred in both sexes (47). Compared with NH whites, binge drinking prevalence is similar in blacks, lower in Asians, and slightly higher in Hispanics (13). By state, prevalence of binge drinking in 2014 ranged from 11% in West Virginia to 25% in North Dakota (45); however, the intensity of binge drinking (the number of drinks per occasion) is highest in the central South and parts of the Midwest (13).
Approximately 4% of cancer cases and 5% of cancer deaths in the United States are attributable to unhealthy diet, including high consumption of red and processed meat and low dietary intake of fruit and vegetables, fiber, and calcium (34). Many Americans do not meet dietary recommendations. For example, daily consumption of ≥3 servings of vegetables and ≥2 servings of fruits was reported by only 16% and 29% of adults in 2015, respectively (48). Similarly, despite a modest increase in leisure physical activity prevalence over the past few decades, 44% of men and 50% of women aged ≥18 years in the United States in 2015 did not meet recommended aerobic and muscle-strengthening activities (38). Prevalence of unhealthy diet and physical inactivity varies across states and is higher among lower socioeconomic groups (38,45,49).
The combination of excess weight, alcohol consumption, unhealthy diet, and physical inactivity accounts for 18% of all cancer cases (14% in men and 22% in women) and 16% of all cancer deaths (15% in men and 17% in women) in the United States (34). More research is needed to increase adherence to comprehensive guidelines on weight control, alcohol, diet, and physical activity. Some measures include family- and school-based interventions, implementation of preventive measures (consultation, screening, and treatment) in the health care system, increasing awareness through education campaigns, and public health laws (such as taxation and reducing marketing of nonessential high-calorie foods and beverages and alcohol) (50-55).
The CDC has recommended a set of strategies and associated measurements that could be used by communities and local governments to promote healthy diet (e.g., through increasing the availability of affordable healthy food and beverages and supporting healthy choices) and physical activity (e.g., through creation of safe environments) and other measures to prevent obesity (e.g., encouraging breastfeeding) (56,57). As behavioral choices are often shaped within the context of the community, these strategies target different settings, including individuals, healthcare providers, communities, schools, public service venues, and worksites (56). Although some states have enacted public health laws to promote healthy diet or physical activity, those laws have largely focused on a few policy areas (e.g., school-based interventions), and all states are far from the implementation of comprehensive strategies to maintain normal weight and substantially reduce excess body weight (58,59).
Recommended strategies by the Community Preventive Services Task Force to prevent excessive alcohol drinking include increasing alcohol tax, regulating alcohol outlet density and days and hours of sale, enforcing laws prohibiting sales to minors and making retail establishments liable for injuries or harms caused by illegal sales/service, maintaining government controls over alcohol sales, and alcohol screening and brief intervention provided in clinical settings or using electronic devices (e.g., cellphones) (46). More information is needed on implementation of these strategies across states and how to measure it (60).
Infections
Nearly 3% of all cancer cases and deaths in both sexes in the United States are attributable to carcinogenic infections with available data, including human papillomavirus (HPV), Helicobacter pylori, hepatitis B and C viruses, and human herpes virus type 8 (34). Persistent infection with HPV accounts for the majority of the cancer cases (2%) attributed to infections (34), despite substantial decreases in cervical cancer incidence rates following the widespread uptake of cervical cancer screening (61). The rise in oropharyngeal and anal cancer rates in the United States has been mainly attributed to an increase in persistent HPV infection in corresponding organs (34,62). Effective preventive measures against cancer are available for HPV (vaccination and cervical cancer screening), hepatitis B virus (vaccination), and hepatitis C virus (curative treatment) infections. However, utilization of these measures is generally suboptimal, with variations across states and socioeconomic groups (48). For example, only 43% of adolescents ages 13–17 years were up to date with HPV vaccination as of 2016, ranging from 27% in Wyoming to 71% in Rhode Island (63). One-time hepatitis C virus testing is recommended for those born in 1945–1965 in the United States (also called “baby boomers”), who account for the majority of chronic HCV infections, but only a small fraction of this population has reported testing (14% in 2015) and even fewer have received treatment (13,64).
Other risk factors
Ultraviolet radiation from sun exposure and indoor tanning contributes to 95% of skin melanomas—or 5% of all cancers excluding non-melanoma skin cancer—in the United States (34). In addition, there are a number of other potentially modifiable risk factors that are generally less common and/or lack nationally representative data, such as radiation other than ultraviolet, air pollution, chemical agents, occupational hazards, and reproductive factors (65,66). However, a few studies have estimated proportion of cancers attributable to some of those risk factors (67-70). For example, an estimated 8,800 lung cancer deaths in the United States in 2012 were attributed to ambient air pollution (70), accounting for approximately 6% of lung cancer deaths in that year (12).
Cancer screening
In the United States, routine screening for early detection of cancers of the cervix, colorectum, and female breast and precancerous lesions of the cervix and colorectum is recommended and has contributed to declining cancer death rates, as well as cervical and colorectal cancer incidence rates, in recent decades (1,61,71,72). Routine lung cancer screening with low-dose spiral computed tomography became recommended in 2013 in the United States for current or former (quit within the last 15 years) heavy smokers. Additional inclusion criteria for screening vary across cancer types (48,61). Previously, screening for prostate cancer using prostate-specific antigen was also recommended, but routine use of this test among asymptomatic men aged ≥75 years since 2008 and for men of all ages since 2012 is no longer recommended by the U.S. Preventive Services Task Force (USPSTF) due to complications associated with over-diagnosis and treatment (48). The American Cancer Society and the American College of Physicians currently support shared decision making regarding the use of prostate-specific antigen testing after receiving information about potential benefits, risks, and uncertainties associated with prostate cancer screening; the USPSTF has a similar proposition in its more recent draft recommendation (48).
From 2000–2015, cancer screening uptake remained stable for breast cancer, increased for colorectal cancer, and slightly declined for cervical cancer (73). In 2015, the proportion of eligible individuals that were up to date with screening consistent with the USPSTF recommendations was 71.5% for female breast cancer, 83.0% for cervical cancer, and 62.4% for colorectal cancer (73). These proportions remain below the Healthy People 2020 targets, which are 81.1% for breast cancer; 93.0% for cervical cancer, and 70.5% for colorectal cancer (73). Lung cancer screening among eligible current and recent heavy smokers has been low (only 3.9% in 2015) (74). In 2015, 34.4% of men 50 years or older had undergone prostate-specific antigen testing within the past year (48).
As expected, there are substantial disparities in participation in screening programs in the United States by indicators of socioeconomic status, such as education level and more importantly, health insurance coverage (48,73). For example, the proportion of individuals who were up to date with screening for breast cancer in 2015 was 76.7% among those with private insurance and 35.3% among those with no insurance; the corresponding proportions were 86.8% and 63.8% for cervical cancer and 65.6% and 25.1% for colorectal cancer (73).
Cancer treatment
Improvements in cancer treatment, availability of an increasing number of new anticancer drugs, and an increase in access to timely treatment has been associated with an increase in survival and decrease in mortality rates for some cancers in the United States (75). Some of the most striking increases in survival have occurred for hematologic and lymphoid malignancies due to advances in treatment. For example, the 5-year relative survival rate for chronic myeloid leukemia increased from 22% in the mid-1970s to 68% in 2007–2013, largely as a result of the introduction of tyrosine kinase inhibitors (1).
For many cancers, however, survival remains relatively poor, even for early stage disease in some cancers. For example, although surgical resection, ablation, and liver transplantation are potentially curative treatments for liver cancer (76), the 5-year relative survival rate in the United States is only 37% for localized, 13% for regional, and 4% for distant stage liver cancer (13). However, some of the new biologic therapies have shown promising results in treating more advanced cases of certain cancers. For example, adjuvant therapy with pembrolizumab or nivolumab (monoclonal antibodies against programmed death 1) has increased progression-free and overall survival in advanced melanoma (77,78). Progress in the selection of appropriate treatment based on molecular characteristics of the tumor (also known as precision or personalized medicine) has also been associated with an increase in efficacy and/or avoidance of unnecessary adverse effects for some cancer patients, including breast cancer cases with certain genetic biomarkers (79). However, despite rapid progress, these new treatments are currently available for only a relatively small group of cancer patients, and in some cases, the increase in the survival is short. For example, sorafenib (an oral multikinase inhibitor) has increased the survival in advanced stage liver cancers for only a few months in average (76). Further, these treatments generally have a high cost, making them less accessible to lower socioeconomic groups (79). Inequality in the receipt of appropriate treatment also exists for more traditional treatments (including surgical excision, radiotherapy, and chemotherapy) across racial/ethnic groups and states (80,81).
Health insurance coverage
The proportion of individuals without health insurance coverage in the United States decreased considerably after 2014, when many provisions of the Affordable Care Act went into effect. For example, the proportion of those without insurance for the entire calendar year declined from 13.3% (or 41.8 million individuals) in 2013 to 8.8% (28.1 million individuals) in 2016 (82). However, the proportion of uninsured individuals is higher in ages <64 years (10.1% vs. 1.2% in older ages in 2016), as U.S. residents aged 65 years or more have near-universal healthcare coverage through Medicare (82). There is also variation in the proportion of uninsured individuals across states. In 2014, the proportion of adults aged 18–64 years without current health insurance coverage ranged from 5.3% in Massachusetts to 29.0% in Texas (83). One of the main factors exacerbating this disparity appears to be differences in state-level Medicaid expansion, which allows individuals earning up to 138% of the federal poverty level to apply for the government-funded health insurance program. In 2014, the proportion of adults aged 18–64 years without current health insurance coverage ranged from 5.3% to 20.6% in Medicaid expansion states, whereas it ranged from 10.4% to 29.0% in non-expansion states (83). By January 1, 2014, 24 states and the District of Columbia had expanded Medicaid eligibility; this number increased to 30 states and the District of Columbia by January 1, 2016 (82). However, the recent repeal of the individual mandate to purchase health insurance (which will go into effect from 2019) and efforts to reduce requirements in the health insurance exchanges are likely to increase the proportion of uninsured individuals or those with less comprehensive insurance plans in the United States in the future (84).
Conclusions
Despite the steady decline in both incidence (male only) and death rates over the past decades, about 1.6 million new cancer cases and 600,000 cancer deaths occur in the United States annually, with more than 40% of these cases/deaths attributed to potentially modifiable risk factors. A decrease in smoking prevalence over the past five decades has resulted in a decline in incidence and death rates of lung cancer and other smoking-related cancers, but smoking remains the major contributor to cancer incidence and mortality in the United States. Excess body weight, unhealthy diet, excessive alcohol drinking, and physical inactivity accounts for a considerable proportion of cancers in the country, second only to smoking. Although the prevalence of certain oncogenic infections (e.g., HCV/HBV) is relatively low in the United States, infections have also contributed to the rise in the occurrence of some cancers, including cancers of the liver (notably among baby boomers), oropharynx, and anus. Comprehensive implementation of successful interventions to curb smoking, excess body weight, and other potentially modifiable risk factors could prevent many cancer cases and avoid morbidity and premature mortality. Optimal participation in recommended cancer screening programs and equitable access to preventive care and treatment could further reduce the burden of cancer in the United States.
Acknowledgments
Funding: This work supported by the Intramural Research Department of the American Cancer Society.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Wanqing Chen) for the series “Global Cancer Burden” published in Annals of Cancer Epidemiology. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ace.2018.08.02). The series “Global Cancer Burden” was commissioned by the editorial office without any funding or sponsorship. A. Jemal serves as the unpaid Honorary Editor-in-Chief of Annals of Cancer Epidemiology. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Jemal A, Ward EM, Johnson CJ, et al. Annual Report to the Nation on the Status of Cancer, 1975-2014, Featuring Survival. J Natl Cancer Inst 2017;109: [Crossref] [PubMed]
- SEER*Stat Database: NAACCR Incidence Data - CiNA Analytic File, 1995-2014 North American Association of Central Cancer Registries 2016; submitted.
- Howlader N, Noone AM, Krapcho M, et al. editors. SEER Cancer Statistics Review, 1975-2014, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2014/, based on November 2016 SEER data submission, posted to the SEER web site, April 2017.
- Johnson CJ, Mariotto AB, Nishri D, et al. Cancer in North America: 2010-2014 Volume Four: Cancer Survival in the United States and Canada 2007-2013. Springfield, IL: North American Association of Central Cancer Registries, Inc., 2017.
- Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 2016;66:271-89. [Crossref] [PubMed]
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence Estimate of Nonmelanoma Skin Cancer (Keratinocyte Carcinomas) in the U.S. Population, 2012. JAMA Dermatol 2015;151:1081-6. [Crossref] [PubMed]
- Jemal A, Ma J, Siegel R, et al. Prostate Cancer Incidence Rates 2 Years After the US Preventive Services Task Force Recommendations Against Screening. JAMA Oncol 2016;2:1657-60. [Crossref] [PubMed]
- Islami F, DeSantis CE, Jemal A. Incidence Trends of Esophageal and Gastric Cancer Subtypes by Race, Ethnicity, and Age in the United States, 1997-2014. Clin Gastroenterol Hepatol 2018; [Epub ahead of print]. [PubMed]
- Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal Cancer Incidence Patterns in the United States, 1974-2013. J Natl Cancer Inst 2017;109: [Crossref] [PubMed]
- Copeland G, Lake A, Firth R, et al. Cancer in North America: 2010-2014. Volume two: Registry-specific cancer incidence in the United States and Canada. Springfield, IL: North American Association of Central Cancer Registries, Inc., 2017.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Islami F, Miller KD, Siegel RL, et al. Disparities in liver cancer occurrence in the United States by race/ethnicity and state. CA Cancer J Clin 2017;67:273-89. [Crossref] [PubMed]
- Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the "Silver Tsunami": Prevalence Trajectories and Comorbidity Burden among Older Cancer Survivors in the United States. Cancer Epidemiol Biomarkers Prev 2016;25:1029-36. [Crossref] [PubMed]
- United States Department of Health and Human Services (US DHHS), Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS), Bridged-Race Population Estimates, United States July 1st resident population by state, county, age, sex, bridged-race, and Hispanic origin. Compiled from 1990-1999 bridged-race intercensal population estimates (released by NCHS on 7/26/2004); revised bridged-race 2000-2009 intercensal population estimates (released by NCHS on 10/26/2012); and bridged-race Vintage 2017 (2010-2017) postcensal population estimates (released by NCHS on 6/27/2018). Available on CDC WONDER Online Database. Available online: http://wonder.cdc.gov/bridged-race-v2017.html, accessed on Jul 30, 2018.
- American Community Survey Briefs. Household Income: 2016. Available online: https://www.census.gov/library/publications/2017/acs/acsbr16-02.html, United States Census Bureau, 2017.
- Li J, Weir HK, Jim MA, et al. Kidney cancer incidence and mortality among American Indians and Alaska Natives in the United States, 1990-2009. Am J Public Health 2014;104:S396-403. [Crossref] [PubMed]
- Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin 2017;67:177-93. [Crossref] [PubMed]
- Pinheiro PS, Callahan KE, Siegel RL, et al. Cancer Mortality in Hispanic Ethnic Groups. Cancer Epidemiol Biomarkers Prev 2017;26:376-82. [Crossref] [PubMed]
- Torre LA, Sauer AM, Chen MS Jr, et al. Cancer statistics for Asian Americans, Native Hawaiians, and Pacific Islanders, 2016:Converging incidence in males and females. CA Cancer J Clin 2016;66:182-202. [Crossref] [PubMed]
- Ma J, Altekruse S, Cosgrove C, et al. Educational Disparities in Mortality Between Adults Aged 50-64 and 66-79 Years, U.S. Am J Prev Med 2017;52:728-34. [Crossref] [PubMed]
- Jemal A, Siegel RL, Ma J, et al. Inequalities in premature death from colorectal cancer by state. J Clin Oncol 2015;33:829-35. [Crossref] [PubMed]
- Lansdorp-Vogelaar I, Kuntz KM, Knudsen AB, et al. Contribution of screening and survival differences to racial disparities in colorectal cancer rates. Cancer Epidemiol Biomarkers Prev 2012;21:728-36. [Crossref] [PubMed]
- Augustus GJ, Ellis NA. Colorectal Cancer Disparity in African Americans: Risk Factors and Carcinogenic Mechanisms. Am J Pathol 2018;188:291-303. [Crossref] [PubMed]
- Daly B, Olopade OI. A perfect storm: How tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J Clin 2015;65:221-38. [Crossref] [PubMed]
- Siegel RL, Sahar L, Robbins A, et al. Where can colorectal cancer screening interventions have the most impact? Cancer Epidemiol Biomarkers Prev 2015;24:1151-6. [Crossref] [PubMed]
- Ross K, Kramer MR, Jemal A. Geographic Inequalities in Progress against Lung Cancer among Women in the United States, 1990-2015. Cancer Epidemiol Biomarkers Prev 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Smith RA, Andrews KS, Brooks D, et al. Cancer screening in the United States, 2018:A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin 2018;68:297-316. [Crossref] [PubMed]
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2006. CA Cancer J Clin 2006;56:106-30. [Crossref] [PubMed]
- Islami F, Ward EM, Jacobs EJ, et al. Potentially preventable premature lung cancer deaths in the USA if overall population rates were reduced to those of educated whites in lower-risk states. Cancer Causes Control 2015;26:409-18. [Crossref] [PubMed]
- Danaei G, Ding EL, Mozaffarian D, et al. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med 2009;6:e1000058 [Crossref] [PubMed]
- Schottenfeld D, Beebe-Dimmer JL, Buffler PA, et al. Current perspective on the global and United States cancer burden attributable to lifestyle and environmental risk factors. Annu Rev Public Health 2013;34:97-117. [Crossref] [PubMed]
- Song M, Giovannucci E. Preventable Incidence and Mortality of Carcinoma Associated With Lifestyle Factors Among White Adults in the United States. JAMA Oncol 2016;2:1154-61. [Crossref] [PubMed]
- Islami F, Goding Sauer A, Miller KD, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin 2018;68:31-54. [Crossref] [PubMed]
- Siegel RL, Jacobs EJ, Newton CC, et al. Deaths Due to Cigarette Smoking for 12 Smoking-Related Cancers in the United States. JAMA Intern Med 2015;175:1574-6. [Crossref] [PubMed]
- Lortet-Tieulent J, Goding Sauer A, Siegel RL, et al. State-Level Cancer Mortality Attributable to Cigarette Smoking in the United States. JAMA Intern Med 2016;176:1792-8. [Crossref] [PubMed]
- Drope J, Liber AC, Cahn Z, et al. Who's still smoking? Disparities in adult cigarette smoking prevalence in the United States. CA Cancer J Clin 2018;68:106-15. [Crossref] [PubMed]
- National Center for Health Statistics. Health, United States, 2016: With chartbook on long-term trends in health. DHHS Publication No. 2017-1232. Hyattsville, MD: U.S. Department of Health and Human Services, 2017.
- American Cancer Society Cancer Action Network. How do you measure up? A progress report on state legislative activity to reduce cancer incidence and mortality, 15th Edition. Available online: https://www.acscan.org/sites/default/files/National%20Documents/HDYMU-2017.pdf, last accessed March 14, 2018.
- Levy DT, Meza R, Zhang Y, et al. Gauging the Effect of U.S. Tobacco Control Policies From 1965 Through 2014 Using SimSmoke. Am J Prev Med 2016;50:535-42. [Crossref] [PubMed]
- Centers for Disease Control and Prevention. Best practices for comprehensive tobacco control programs - 2014. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014.
- Ogden CL, Carroll MD. Prevalence of overweight, obesity, and extreme obesity among adults: United States, trends 1960–1962 through 2007–2008. National Center for Health Statistics, Health E-Stats. 2010.
- Flegal KM, Kruszon-Moran D, Carroll MD, et al. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA 2016;315:2284-91. [Crossref] [PubMed]
- Ogden CL, Fakhouri TH, Carroll MD, et al. Prevalence of Obesity Among Adults, by Household Income and Education - United States, 2011-2014. MMWR Morb Mortal Wkly Rep 2017;66:1369-73. [Crossref] [PubMed]
- Gamble S, Mawokomatanda T, Xu F, et al. Surveillance for Certain Health Behaviors and Conditions Among States and Selected Local Areas - Behavioral Risk Factor Surveillance System, United States, 2013 and 2014. MMWR Surveill Summ 2017;66:1-144. [Crossref] [PubMed]
- National Center for Chronic Disease Prevention and Health Promotion. Excessive alcohol use - Preventing a leading risk for death, disease, and injury. 2016.
- Han BH, Moore AA, Sherman S, et al. Demographic trends of binge alcohol use and alcohol use disorders among older adults in the United States, 2005-2014. Drug Alcohol Depend 2017;170:198-207. [Crossref] [PubMed]
- Sauer AG, Siegel RL, Jemal A, et al. Updated Review of Prevalence of Major Risk Factors and Use of Screening Tests for Cancer in the United States. Cancer Epidemiol Biomarkers Prev 2017;26:1192-208. [Crossref] [PubMed]
- Lee-Kwan SH, Moore LV, Blanck HM, et al. Disparities in State-Specific Adult Fruit and Vegetable Consumption - United States, 2015. MMWR Morb Mortal Wkly Rep 2017;66:1241-7. [Crossref] [PubMed]
- Cauchi D, Glonti K, Petticrew M, et al. Environmental components of childhood obesity prevention interventions: an overview of systematic reviews. Obes Rev 2016;17:1116-30. [Crossref] [PubMed]
- US Preventive Services Task Force. Behavioral Counseling to Promote a Healthful Diet and Physical Activity for Cardiovascular Disease Prevention in Adults Without Cardiovascular Risk Factors: US Preventive Services Task Force Recommendation Statement. JAMA 2017;318:167-74. [Crossref] [PubMed]
- US Preventive Services Task Force. Screening for Obesity in Children and Adolescents: US Preventive Services Task Force Recommendation Statement. JAMA 2017;317:2417-26. [Crossref] [PubMed]
- Moyer VAU. S. Preventive Services Task Force. Screening for and management of obesity in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2012;157:373-8. [Crossref] [PubMed]
- Shuval K, Leonard T, Drope J, et al. Physical activity counseling in primary care: Insights from public health and behavioral economics. CA Cancer J Clin 2017;67:233-44. [Crossref] [PubMed]
- Dunstone K, Brennan E, Slater MD, et al. Alcohol harm reduction advertisements: a content analysis of topic, objective, emotional tone, execution and target audience. BMC Public Health 2017;17:312. [Crossref] [PubMed]
- Khan LK, Sobush K, Keener D, et al. Recommended community strategies and measurements to prevent obesity in the United States. MMWR Recomm Rep 2009;58:1-26. [PubMed]
- Centers for Disease Control and Prevention. State initiatives supporting healthier food retail: An overview of the national landscape. Division of Nutrition, Physical Activity, and Obesity. Available online: https://www.cdc.gov/obesity/downloads/healthier_food_retail.pdf, last accessed: March 26, 2018.
- Centers for Disease Control and Prevention. Chronic Disease State Policy Tracking System. Available online: https://nccd.cdc.gov/cdphppolicysearch/default.aspx#, last accessed: March 14, 2018.
- Pomeranz JL, Siddiqi A, Bolanos GJ, et al. Consolidated state political party control and the enactment of obesity-related policies in the United States. Prev Med 2017;105:397-403. [Crossref] [PubMed]
- Centers for Disease Control and Prevention. Guide for measuring alcohol outlet density. Atlanta, GA: CDC, US Dept of Health and Human Services, 2017.
- Smith RA, Andrews KS, Brooks D, et al. Cancer screening in the United States, 2017: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin 2017;67:100-21. [Crossref] [PubMed]
- Islami F, Ferlay J, Lortet-Tieulent J, et al. International trends in anal cancer incidence rates. Int J Epidemiol 2017;46:924-38. [PubMed]
- Walker TY, Elam-Evans LD, Singleton JA, et al. National, Regional, State, and Selected Local Area Vaccination Coverage Among Adolescents Aged 13-17 Years - United States, 2016. MMWR Morb Mortal Wkly Rep 2017;66:874-82. [Crossref] [PubMed]
- Jemal A, Fedewa SA. Recent Hepatitis C Virus Testing Patterns Among Baby Boomers. Am J Prev Med 2017;53:e31-e33. [Crossref] [PubMed]
- Cogliano VJ, Baan R, Straif K, et al. Preventable exposures associated with human cancers. J Natl Cancer Inst 2011;103:1827-39. [Crossref] [PubMed]
- Boffetta P, Islami F. The contribution of molecular epidemiology to the identification of human carcinogens: current status and future perspectives. Ann Oncol 2013;24:901-8. [Crossref] [PubMed]
- Plummer M, de Martel C, Vignat J, et al. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health 2016;4:e609-16. [Crossref] [PubMed]
- Purdue MP, Hutchings SJ, Rushton L, et al. The proportion of cancer attributable to occupational exposures. Ann Epidemiol 2015;25:188-92. [Crossref] [PubMed]
- Bartick MC, Schwarz EB, Green BD, et al. Suboptimal breastfeeding in the United States: Maternal and pediatric health outcomes and costs. Matern Child Nutr 2017;13: [Crossref] [PubMed]
- World Health Organization. Ambient air pollution: A global assessment of exposure and burden of disease. Geneva, Switzerland: WHO Press, 2016.
- Lin JS, Piper MA, Perdue LA, et al. Screening for Colorectal Cancer: A Systematic Review for the US Preventive Services Task Force. Rockville (MD): 2016.
- Plevritis SK, Munoz D, Kurian AW, et al. Association of Screening and Treatment With Breast Cancer Mortality by Molecular Subtype in US Women, 2000-2012. JAMA 2018;319:154-64. [Crossref] [PubMed]
- White A, Thompson TD, White MC, et al. Cancer Screening Test Use - United States, 2015. MMWR Morb Mortal Wkly Rep 2017;66:201-6. [Crossref] [PubMed]
- Jemal A, Fedewa SA. Lung Cancer Screening With Low-Dose Computed Tomography in the United States-2010 to 2015. JAMA Oncol 2017;3:1278-81. [Crossref] [PubMed]
- American Society of Clinical Oncology. The State of Cancer Care in America, 2017: A Report by the American Society of Clinical Oncology. J Oncol Pract 2017;13:e353-e394. [Crossref] [PubMed]
- Bruix J, Reig M, Sherman M. Evidence-Based Diagnosis, Staging, and Treatment of Patients With Hepatocellular Carcinoma. Gastroenterology 2016;150:835-53. [Crossref] [PubMed]
- Weber J, Mandala M, Del Vecchio M, et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N Engl J Med 2017;377:1824-35. [Crossref] [PubMed]
- Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N Engl J Med 2018;378:1789-801. [Crossref] [PubMed]
- Kurnit KC, Dumbrava EEI, Litzenburger B, et al. Precision Oncology Decision Support: Current Approaches and Strategies for the Future. Clin Cancer Res 2018;24:2719-31. [Crossref] [PubMed]
- Sineshaw HM, Wu XC, Flanders WD, et al. Variations in Receipt of Curative-Intent Surgery for Early-Stage Non-Small Cell Lung Cancer (NSCLC) by State. J Thorac Oncol 2016;11:880-9. [Crossref] [PubMed]
- Sineshaw HM, Ng K, Flanders WD, et al. Factors That Contribute to Differences in Survival of Black vs White Patients With Colorectal Cancer. Gastroenterology 2018;154:906-915.e7. [Crossref] [PubMed]
- Barnett JC, Berchick ER. Current Population Reports, P60-260, Health insurance coverage in the United States: 2016. Washington, DC: U.S. Government Printing Office, 2017.
- Okoro CA, Zhao G, Fox JB, et al. Surveillance for Health Care Access and Health Services Use, Adults Aged 18-64 Years - Behavioral Risk Factor Surveillance System, United States, 2014. MMWR Surveill Summ 2017;66:1-42. [Crossref] [PubMed]
- Rice T, Unruh LY, van Ginneken E, et al. Universal coverage reforms in the USA: From Obamacare through Trump. Health Policy 2018;122:698-702. [Crossref] [PubMed]
Cite this article as: Islami F, Miller KD, Jemal A. Cancer burden in the United States—a review. Ann Cancer Epidemiol 2018;2:1.